Question: mol For a liquid-phase reaction A => B, with rate law rA = k C - carried out adiabatically: cal HA (298K) = -10000 cal
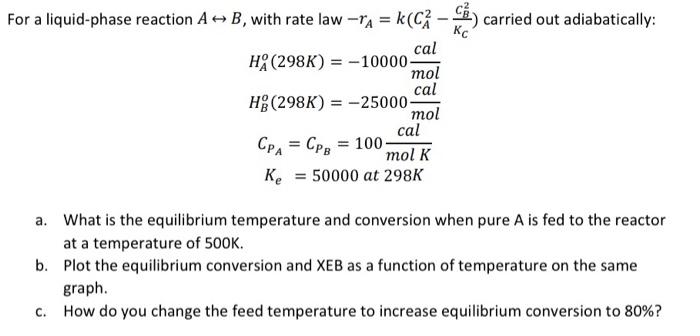
mol For a liquid-phase reaction A => B, with rate law rA = k C - carried out adiabatically: cal HA (298K) = -10000 cal H (298K) = -25000 mol cal CPA = Cpg = 100- mol K K = 50000 at 298K a. What is the equilibrium temperature and conversion when pure A is fed to the reactor at a temperature of 500K. b. Plot the equilibrium conversion and XEB as a function of temperature on the same graph. How do you change the feed temperature to increase equilibrium conversion to 80%? C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


