Question: Molecular Equation: - Write each reactant and product in its own box - Include stoichiometric coefficients and phase symbols - If no reaction occurs in
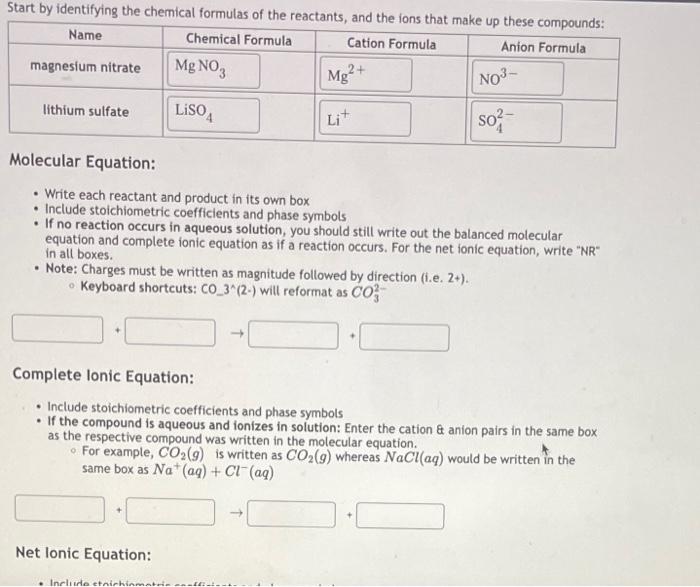
Molecular Equation: - Write each reactant and product in its own box - Include stoichiometric coefficients and phase symbols - If no reaction occurs in aqueous solution, you should still write out the balanced molecular equation and complete ionic equation as if a reaction occurs. For the net ionic equation, write "NR" in all boxes. - Note: Charges must be written as magnitude followed by direction (i.e. 2+ ). - Keyboard shortcuts: CO(2) will reformat as CO32 Complete Ionic Equation: - Include stoichiometric coefficients and phase symbols - If the compound is aqueous and ionizes in solution: Enter the cation \& anion pairs in the same box as the respective compound was written in the molecular equation. - For example, CO2(g) is written as CO2(g) whereas NaCl(aq) would be written in the same box as Na+(aq)+Cl(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


