Question: please explain how to do the molecular and ionic equation as easy as possible The reaction between magnesium nitrate and sodium hydroxide Start bv identifvine
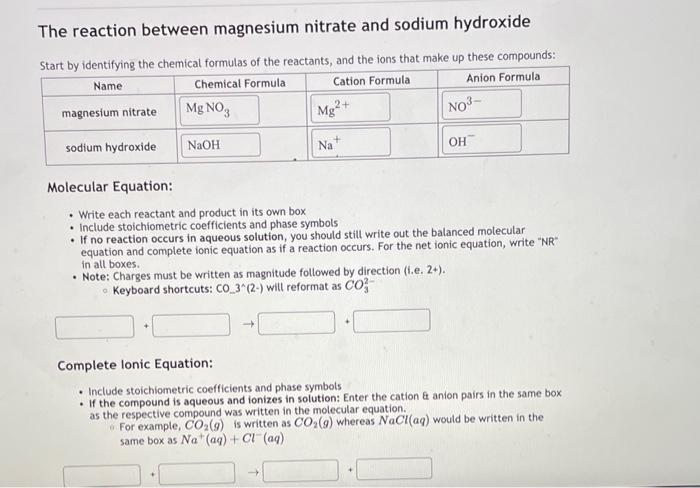
The reaction between magnesium nitrate and sodium hydroxide Start bv identifvine the chemical formulas of the reactants, and the ions that make up these compounds: Molecular Equation: - Write each reactant and product in its own box - Include stoichiometric coefficients and phase symbols - If no reaction occurs in aqueous solution, you should still write out the balanced molecular equation and complete ionic equation as if a reaction occurs. For the net ionic equation, write "NR in all boxes. - Note: Charges must be written as magnitude followed by direction (i.e. 2+ ). Keyboard shortcuts: CO33 (2-) will reformat as CO32 Complete Ionic Equation: - Include stoichiometric coefficients and phase symbols - If the compound is aqueous and ionizes in solution: Enter the cation anion pairs in the same box as the respective compound was written in the molecular equation. For example, CO2(g) is written as CO2(g) whereas NaCl(aq) would be written in the same box as Na+(aq)+Cl(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


