Question: Moving to another question will save the response 1 1 1 Question 13 5 points A reaction has an activation energy of 52 kJ mol
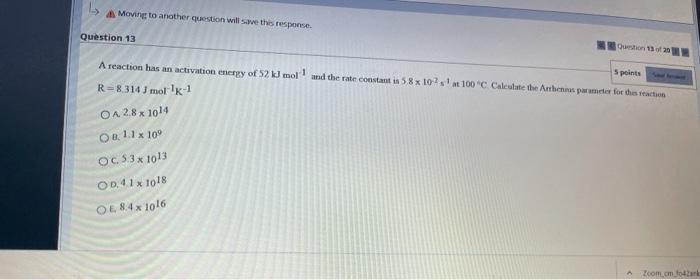
Moving to another question will save the response 1 1 1 Question 13 5 points A reaction has an activation energy of 52 kJ mol and the rate constant in 58 x 102 100C Calculate the Arthens parce for the reaction R-8314) mol-1 O4 28x1014 Oa 1.1 x 10 OC 53x1013 00.41 x 1018 OL841016 Zoom.com for
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


