Question: ? ? n p G f ( products ) - ? ? n r G f ( reactants ) and n r represent the stoichiometric
products reactants
and represent the stoichiometric coefficients in sed chemical equation for the reactants and products ely.
Part B
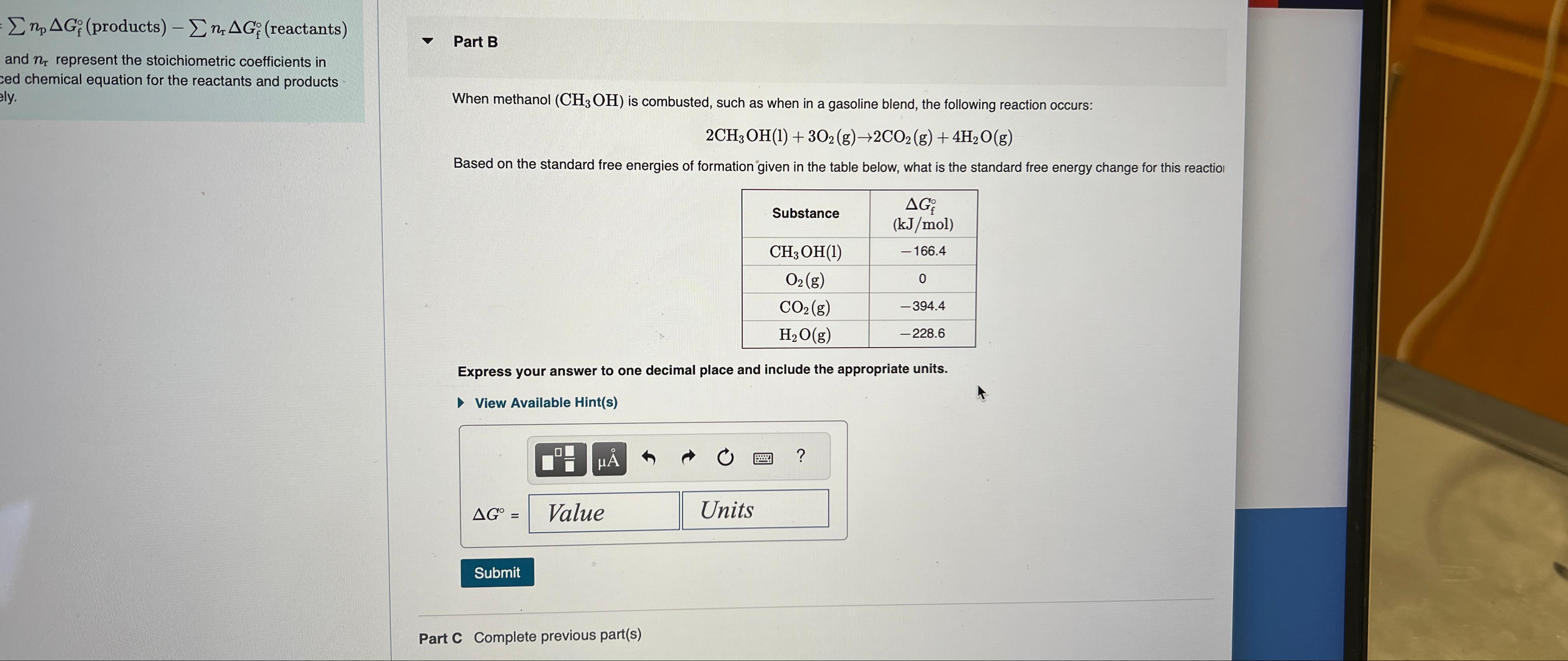
When methanol is combusted, such as when in a gasoline blend, the following reaction occurs:
Based on the standard free energies of formation given in the table below, what is the standard free energy change for this reactiol
tableSubstancetable
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


