Question: Name the following compound systematically. Enter your answers separated by a comma. How many sp, sp2, and sp3 hybridized carbons are in the following molecule?

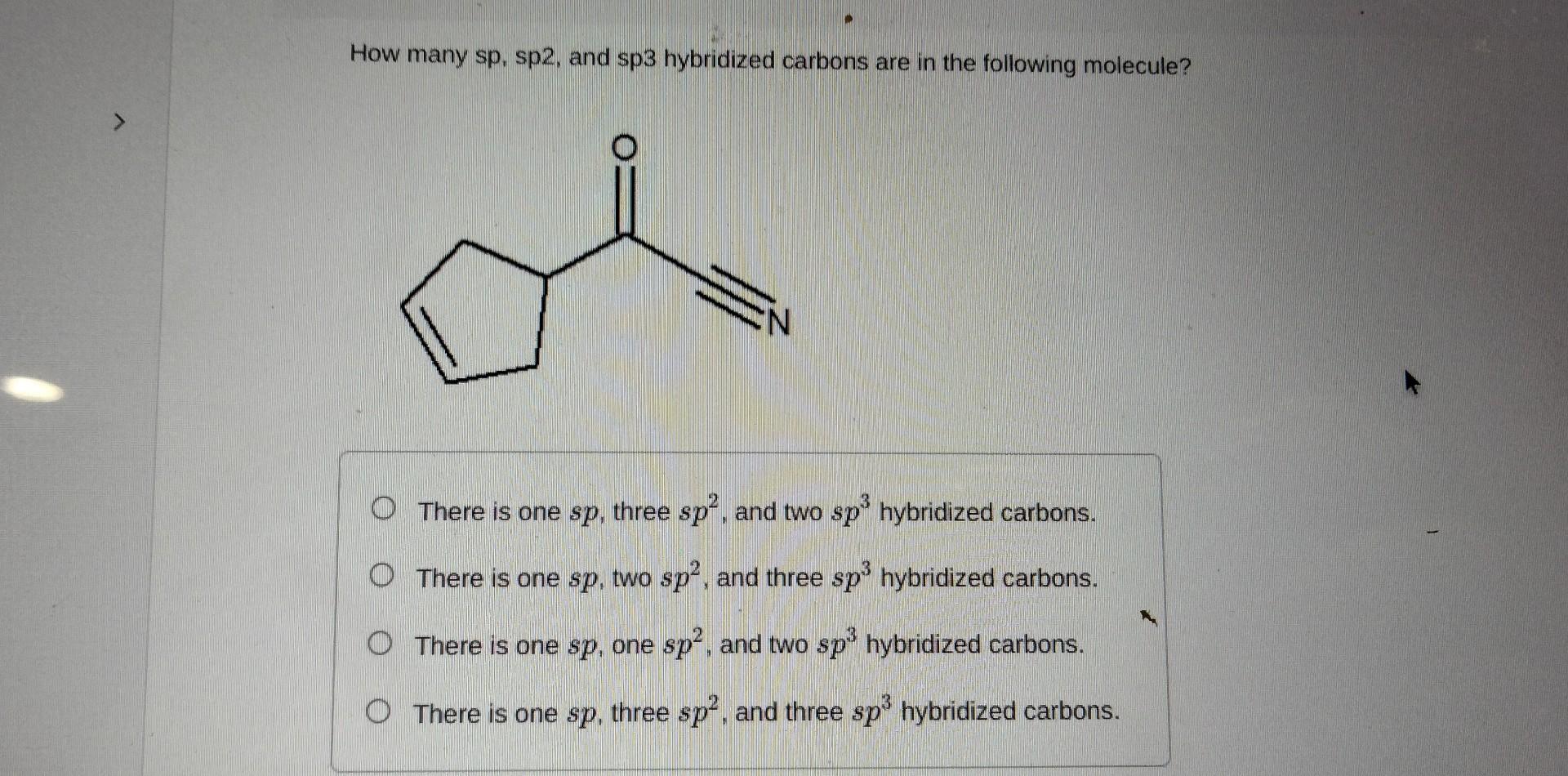

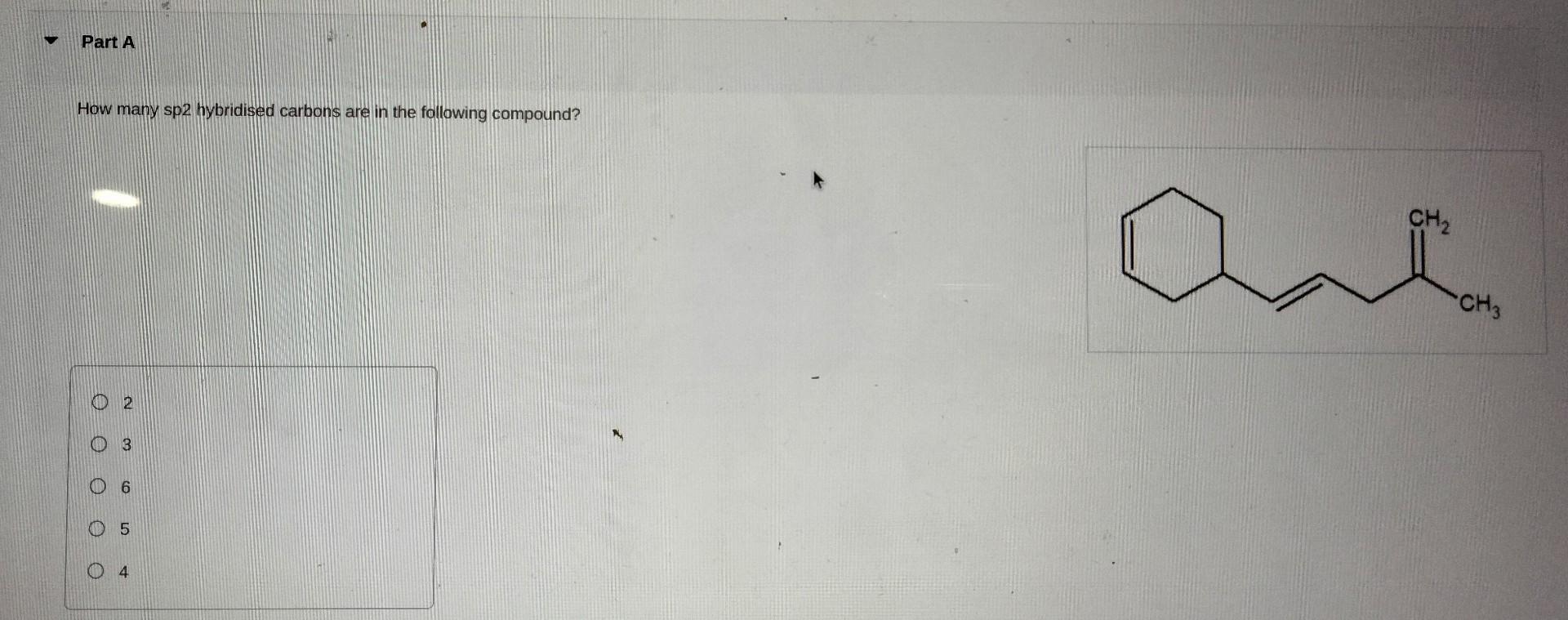
Name the following compound systematically. Enter your answers separated by a comma. How many sp, sp2, and sp3 hybridized carbons are in the following molecule? There is one sp, three sp2, and two sp3 hybridized carbons. There is one sp, two sp2, and three sp3 hybridized carbons. There is one sp, one sp2, and two sp3 hybridized carbons. There is one sp, three sp2, and three sp3 hybridized carbons. Name the following compound systematical|y. How many sp2 hybridised carbons are in the following compound
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


