Question: Name - When nonmetals chemically bond they do so by sharing electrons. The bond is called a covalent bond. - In a covalent bond, a
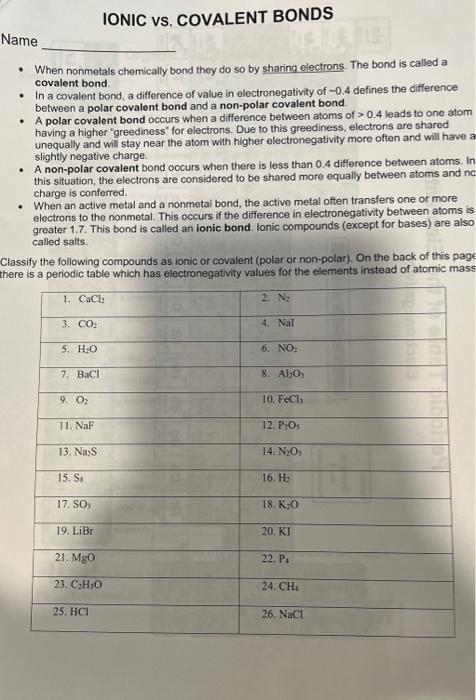
Name - When nonmetals chemically bond they do so by sharing electrons. The bond is called a covalent bond. - In a covalent bond, a difference of value in electronegativity of 0.4 defines the difference between a polar covalent bond and a non-polar covalent bond. - A polar covalent bond occurs when a difference between atoms of >0.4 leads to one atom having a higher "greediness" for electrons. Due to this greediness, electrons are shared unequally and will stay near the atom with higher electronegativity more often and will have a slightly negative charge. - A non-polar covalent bond occurs when there is less than 0.4 difference between atoms, In this situation, the electrons are considered to be shared more equally between atoms and no charge is conferred. - When an active metal and a nonmetal bond, the active metal often transfers one or more electrons to the nonmetal. This occurs if the difference in electronegativity between atoms is greater 1.7. This bond is called an ionic bond. Ionic compounds (except for bases) are also called salts. Classify the following compounds as ionic or covalent (polar or non-polar). On the back of this page there is a periodic table which has electronegativity values for the elements instead of atomic mass
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


