Question: Write down four different covalent compounds that could form, and indicate why they are covalent based on the electronegativity differences. Part III: Number of Bonds
Write down four different covalent compounds that could form, and indicate why they are covalent based on the electronegativity differences.
Part III: Number of Bonds
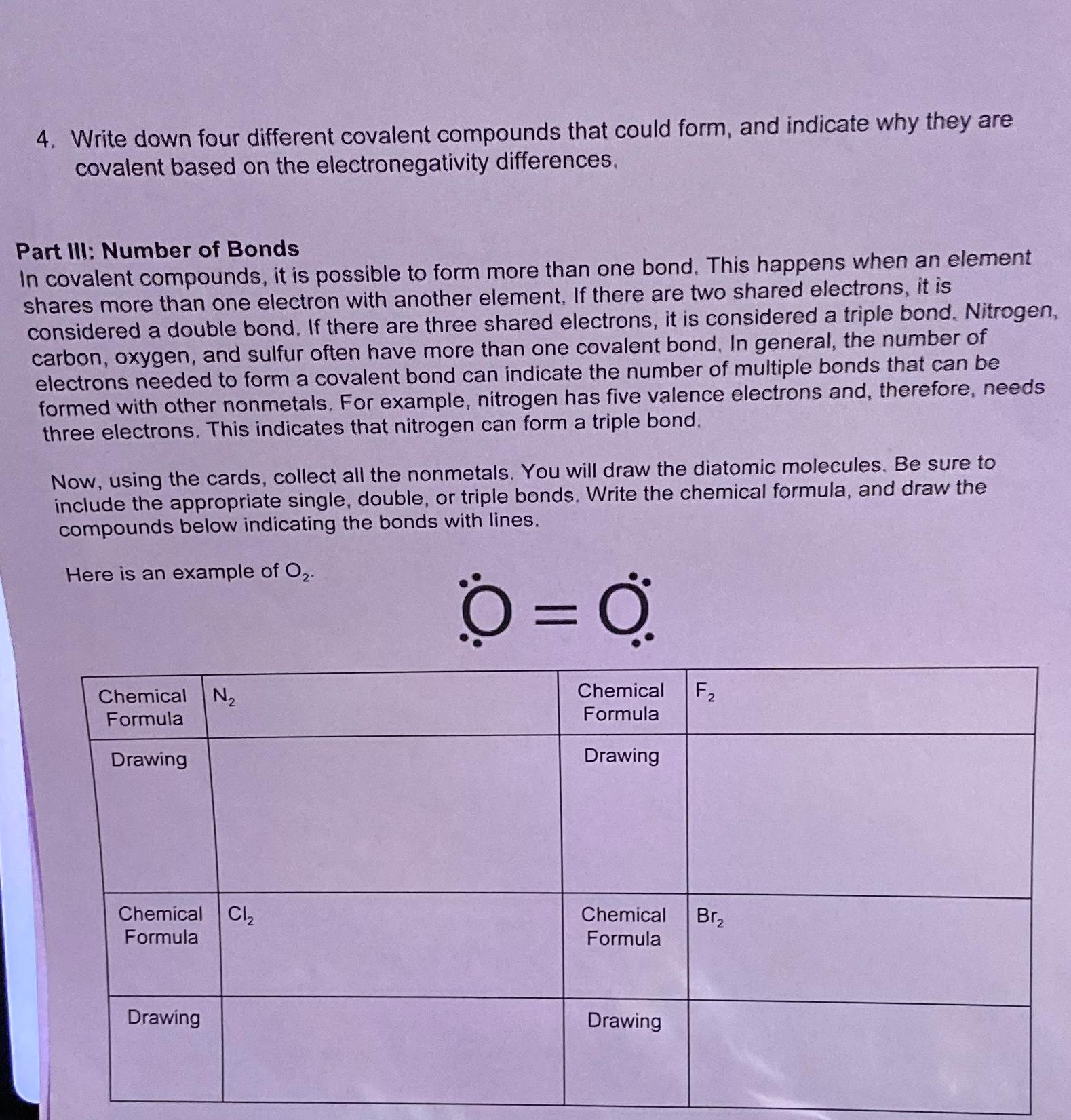
In covalent compounds, it is possible to form more than one bond. This happens when an element shares more than one electron with another element. If there are two shared electrons, it is considered a double bond. If there are three shared electrons, it is considered a triple bond. Nitrogen, carbon, oxygen, and sulfur often have more than one covalent bond, In general, the number of electrons needed to form a covalent bond can indicate the number of multiple bonds that can be formed with other nonmetals. For example, nitrogen has five valence electrons and, therefore, needs three electrons. This indicates that nitrogen can form a triple bond.
Now, using the cards, collect all the nonmetals. You will draw the diatomic molecules. Be sure to include the appropriate single, double, or triple bonds. Write the chemical formula, and draw the compounds below indicating the bonds with lines.
Here is an example of
tabletableChemicalFormulatableChemicalFormula
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


