Question: need help! 1. Explain how infra-red spectroscopy can distinguish between acetone (CH5COCH3) and ethanol (CH3CH2OH). Draw the structure of 2-propanol. a) How many sets of
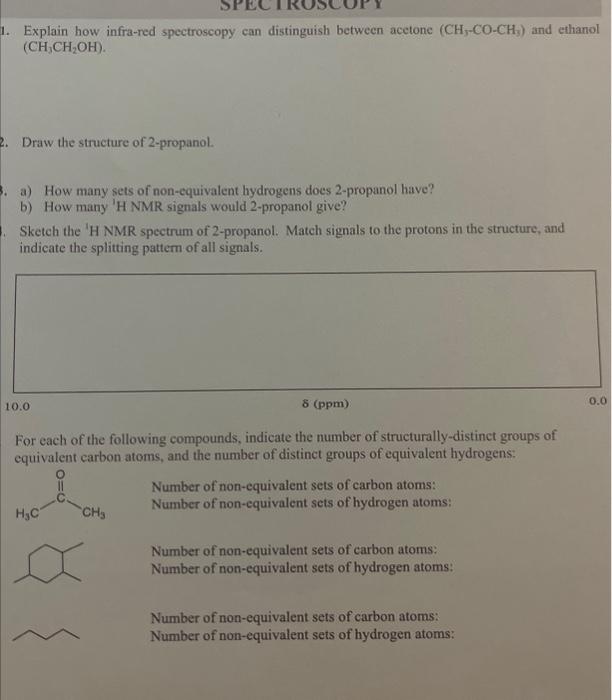
1. Explain how infra-red spectroscopy can distinguish between acetone (CH5COCH3) and ethanol (CH3CH2OH). Draw the structure of 2-propanol. a) How many sets of non-equivalent hydrogens does 2-propanol have? b) How many 'H NMR signals would 2-propanol give? Sketch the 'H NMR spectrum of 2-propanol. Match signals to the protons in the structure, and indicate the splitting pattern of all signals. For each of the following compounds, indicate the number of structurally-distinct groups of equivalent carbon atoms, and the number of distinct groups of equivalent hydrogens: Number of non-equivalent sets of carbon atoms: Number of non-equivalent sets of hydrogen atoms: Number of non-equivalent sets of carbon atoms: Number of non-equivalent sets of hydrogen atoms: Number of non-equivalent sets of carbon atoms: Number of non-equivalent sets of hydrogen atoms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


