Question: need help asap. thanks Experimental data collected Standards absorbance (1) = 0.015, (2) = 0.0078, (3) = 0.0039, (4) = 0.00076 Trials absorbance (1) =
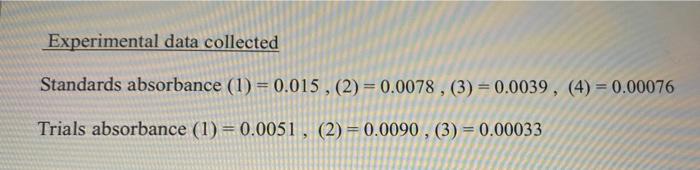
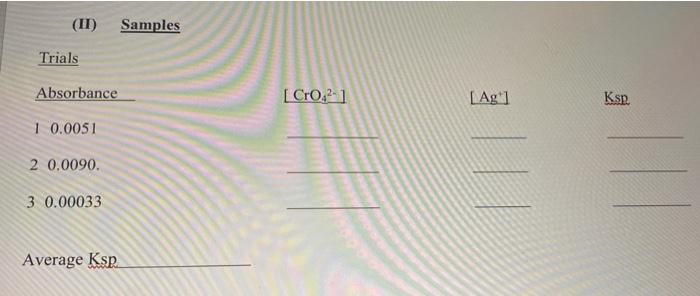

Experimental data collected Standards absorbance (1) = 0.015, (2) = 0.0078, (3) = 0.0039, (4) = 0.00076 Trials absorbance (1) = 0.0051, (2) = 0.0090, (3) = 0.00033 (II) Samples Trials Absorbance [Cro.2 1 Ag 1 Ksp. 1 0.0051 2 0.0090. | | | 3 0.00033 Average Ksp Post-lab (a) Write the solubility (Ksp) expression for silver chromate Show Ksp calculation for trial 1 (b) Finding the solubility of silver chromate in silver nitrate solution: Use the average Kn (1) What would be the molar solubility of silver chromate in 0.15 M AgNO,? ( need to create an ICE table ) (2) What would be the molar solubility of silver chromate in water (3) What is the reason for this difference
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


