Question: need help ik quest 5 and 6 please Question 5 (a) Give the full ionic equation and the net ionic equation for the following precipitation
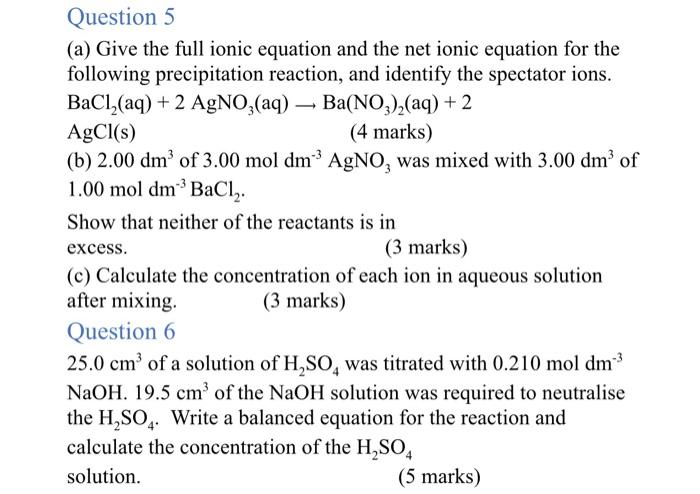
Question 5 (a) Give the full ionic equation and the net ionic equation for the following precipitation reaction, and identify the spectator ions. BaCl2(aq)+2AgNO3(aq)Ba(NO3)2(aq)+2AgCl(s)(4marks) (b) 2.00dm3 of 3.00moldm3AgNO3 was mixed with 3.00dm3 of 1.00moldm3BaCl2. Show that neither of the reactants is in excess. (3 marks) (c) Calculate the concentration of each ion in aqueous solution after mixing. (3 marks) Question 6 25.0cm3 of a solution of H2SO4 was titrated with 0.210moldm3 NaOH.19.5cm3 of the NaOH solution was required to neutralise the H2SO4. Write a balanced equation for the reaction and calculate the concentration of the H2SO4 solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


