Question: NEED HELP WITH C, D, AND E! Consider a particle in a one-dimensional box. For a box of length 1nm, what is the probability of
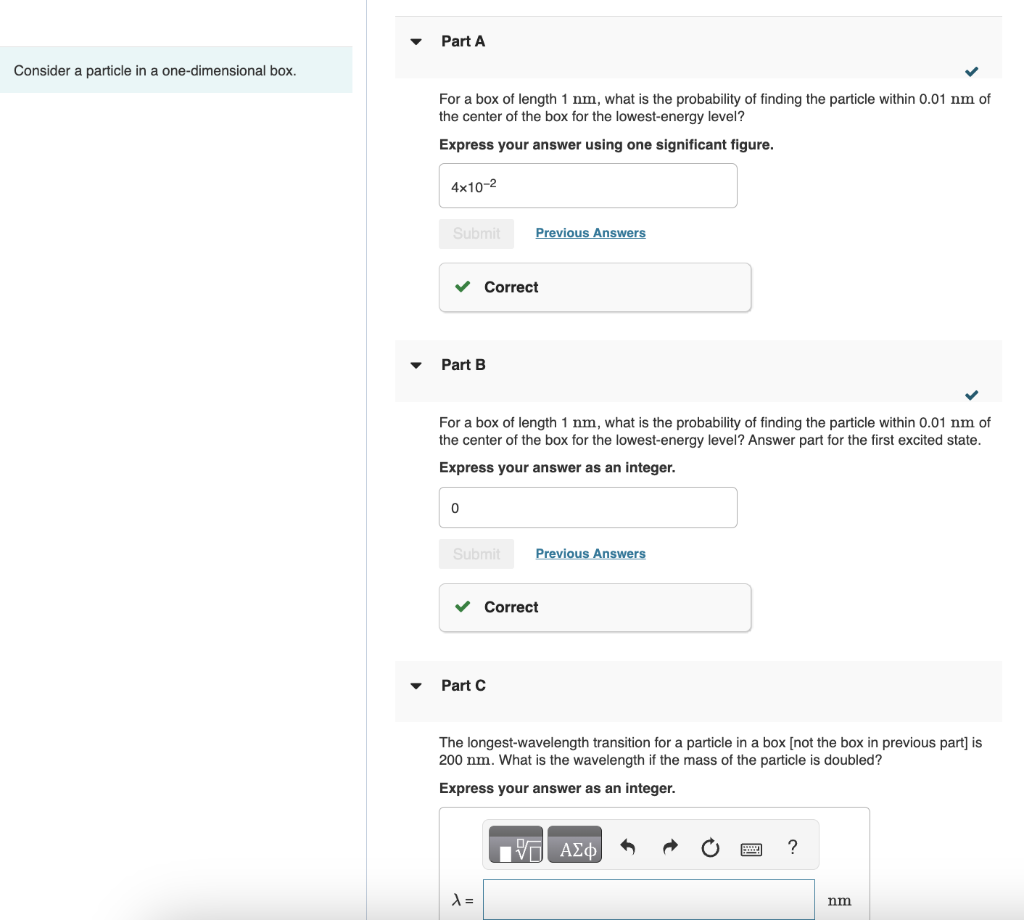
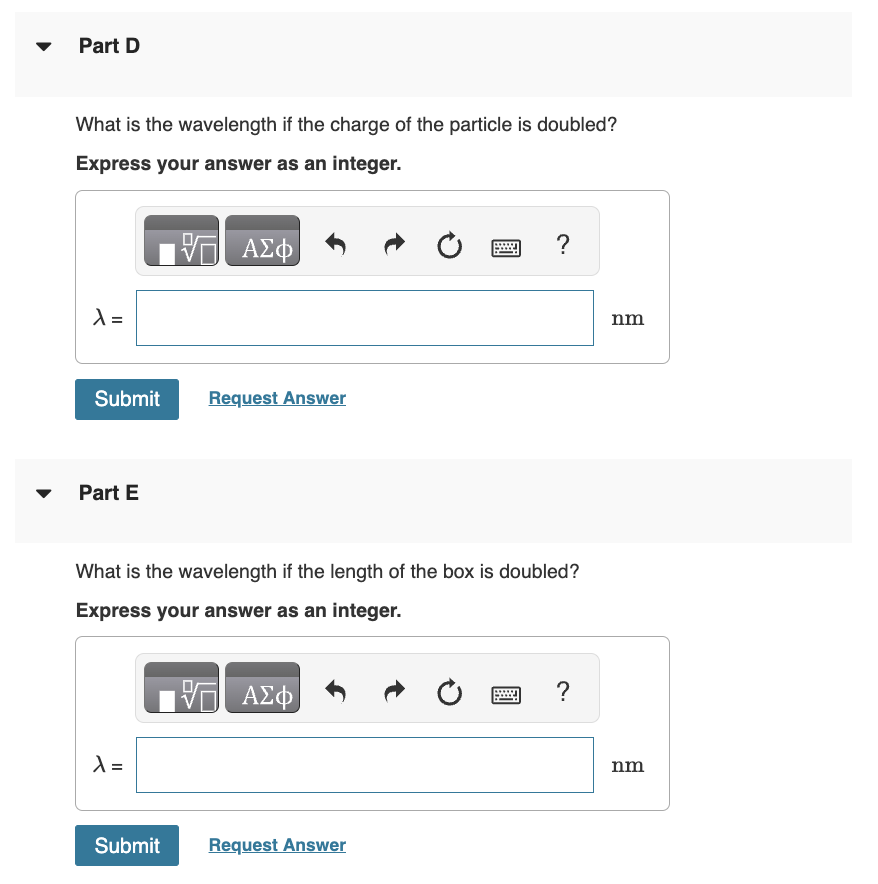 NEED HELP WITH C, D, AND E!
NEED HELP WITH C, D, AND E!
Consider a particle in a one-dimensional box. For a box of length 1nm, what is the probability of finding the particle within 0.01nm of the center of the box for the lowest-energy level? Express your answer using one significant figure. Part B For a box of length 1nm, what is the probability of finding the particle within 0.01nm of the center of the box for the lowest-energy level? Answer part for the first excited state. Express your answer as an integer. Part C The longest-wavelength transition for a particle in a box [not the box in previous part] is 200nm. What is the wavelength if the mass of the particle is doubled? Express your answer as an integer. What is the wavelength if the charge of the particle is doubled? Express your answer as an integer. Part E What is the wavelength if the length of the box is doubled? Express your answer as an integer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


