Question: need help with number 1 and 3, show your work please 1) The molar enthalpy for a particular pure chemical substance at a constant pressure
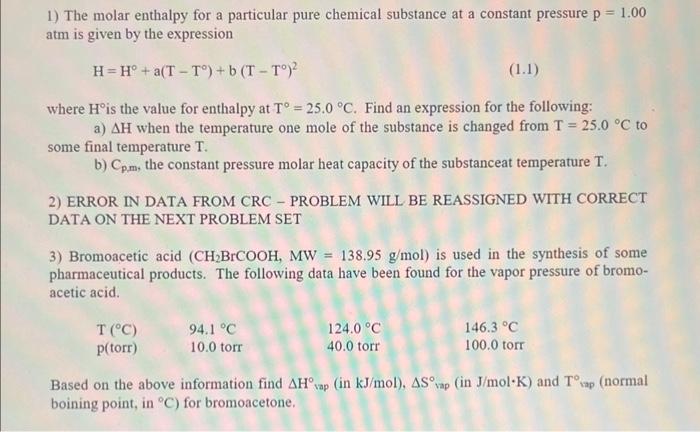
1) The molar enthalpy for a particular pure chemical substance at a constant pressure p=1.00 atm is given by the expression H=H+a(TT)+b(TT)2 where H is the value for enthalpy at T=25.0C. Find an expression for the following: a) H when the temperature one mole of the substance is changed from T=25.0C to some final temperature T. b) Cp,m, the constant pressure molar heat capacity of the substanceat temperature T. 2) ERROR IN DATA FROM CRC - PROBLEM WILL BE REASSIGNED WITH CORRECT DATA ON THE NEXT PROBLEM SET 3) Bromoacetic acid (CH2BrCOOH,MW=138.95g/mol) is used in the synthesis of some pharmaceutical products. The following data have been found for the vapor pressure of bromoacetic acid. Based on the above information find H vap (in kJ/mol ), S vap (in J/molK ) and T iap (normal boining point, in C ) for bromoacetone
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


