Question: Need help with these 3 problems pls help You wish to make a 0.191M perchloric acid solution from a stock solution of 3.00M perchloric acid.
Need help with these 3 problems pls help 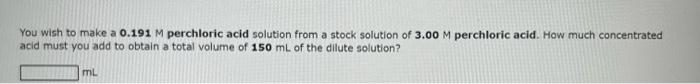





You wish to make a 0.191M perchloric acid solution from a stock solution of 3.00M perchloric acid. How much concentrated acid must you add to obtain a total volume of 150mL of the dillute solution? In the laboratory, a student dilutes 10.9mL of a 10.3M perchloric acid solution to a total volume of 200.0mL. What is the concentration of the diluted solution? Concentration = M How many miliiliters of 11.9M perchloric acid solution should be used to prepare 3.50Lof0.500MHClO ? mL
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


