Question: use the References to access important values if needed for this question. You wish to make a 0.113 M perchloric acid solution from a stock
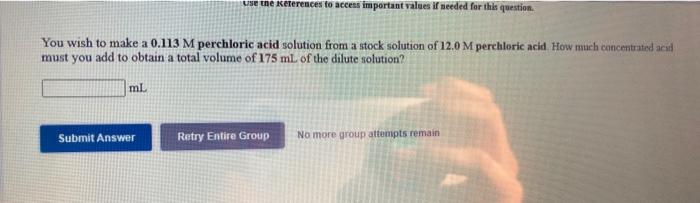

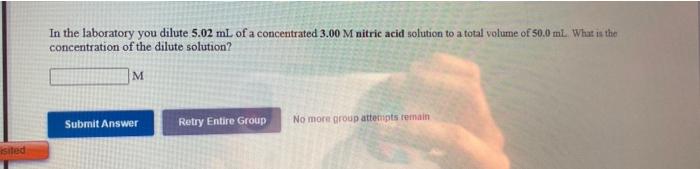
use the References to access important values if needed for this question. You wish to make a 0.113 M perchloric acid solution from a stock solution of 12.0 M perchloric acid How much concentrated acid must you add to obtain a total volume of 175 mL of the dilute solution? ml Submit Answer Retry Entire Group No more group attempts remain You wish to make a 0.162 M nitric acid solution from a stock solution of 3.00 M nitric acid, How much concentrated acid must you add to obtain a total volume of 75.0 mL of the dilute solution? ml In the laboratory you dilute 5.02 mL of a concentrated 3.00 M nitric acid solution to a total volume of 50.0 ml. What is the concentration of the dilute solution? M Submit Answer Retry Entire Group No more group attempts remain isited
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


