Question: need help with this the only information I have is initial titration volume= 72.50 mL final titration volume = 94.50 mL * this is one
need help with this the only information I have is initial titration volume= 72.50 mL final titration volume = 94.50 mL
* this is one question with various parts so please solve them*

the information above is the only information I have
what more information I can give when this is all of it
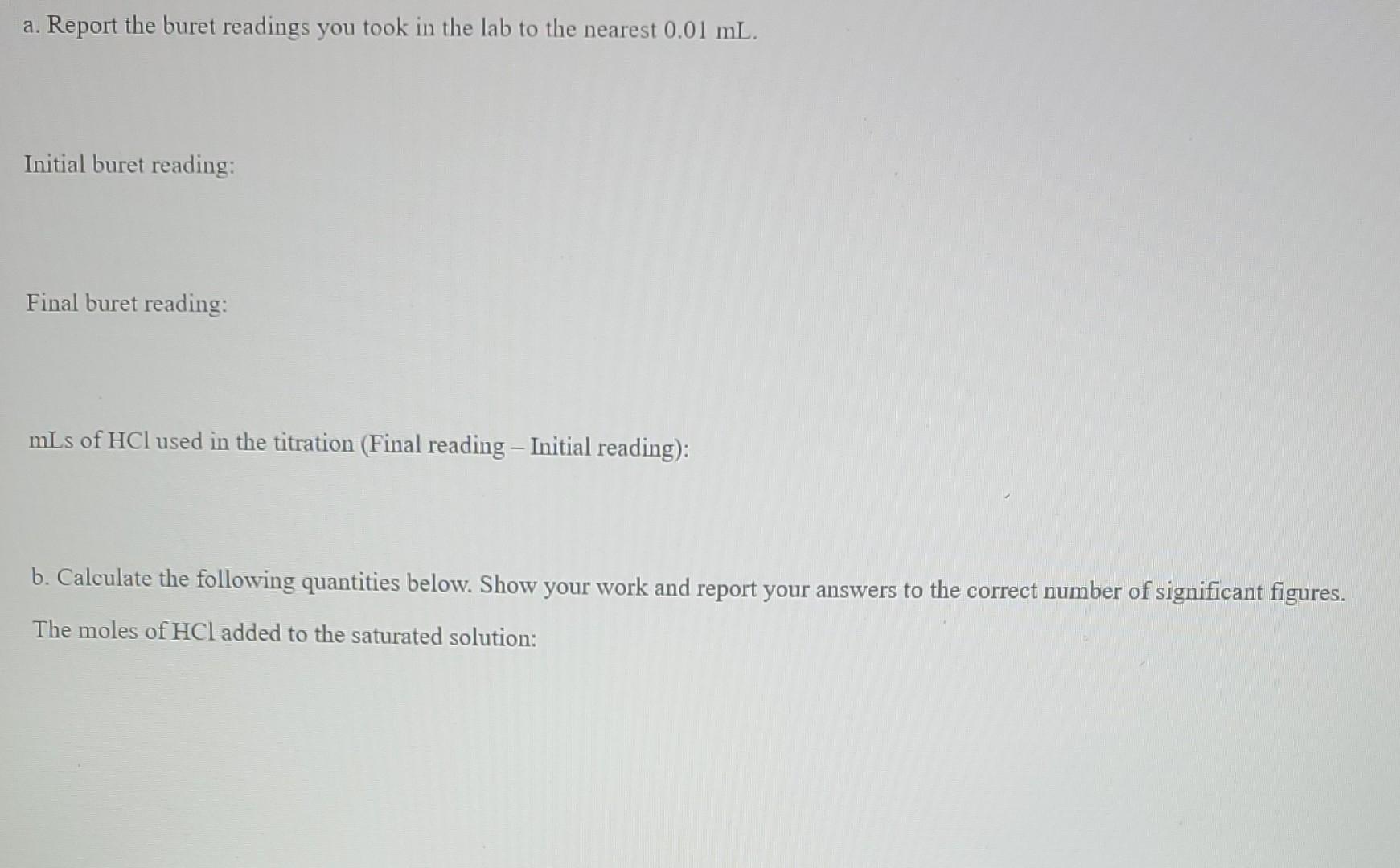
a. Report the buret readings you took in the lab to the nearest 0.01 mL. Initial buret reading: Final buret reading: mLs of HCl used in the titration (Final reading - Initial reading): b. Calculate the following quantities below. Show your work and report your answers to the correct number of significant figures. The moles of HCl added to the saturated solution: The moles of CO32- in the saturated solution: The concentration of CO32- in the saturated solution (in molarity)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


