Question: Needs explanation and steps. Please and thank you In each case, the same ideal gas undergoes a thermodynamic process starting in the same state (same
Needs explanation and steps. Please and thank you
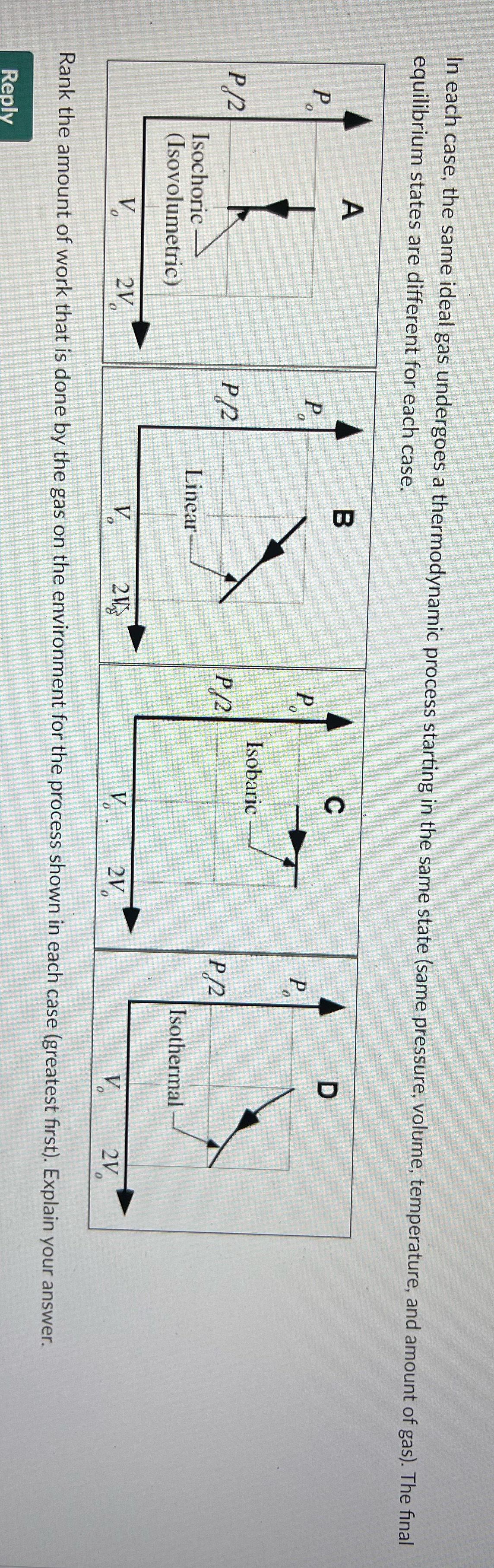
In each case, the same ideal gas undergoes a thermodynamic process starting in the same state (same pressure, volume, temperature, and amount of gas). The final equilibrium states are different for each case. O Isobaric P /2 P/2 Isochoric Linear Isothermal (Isovolumetric) A Rank the amount of work that is done by the gas on the environment for the process shown in each case (greatest first). Explain your answer. Reply
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


