Question: Nitrogen adsorption experiment was conducted to determine the surface area of 1.0g sample of silica gel and results obtained are shown in the table below.
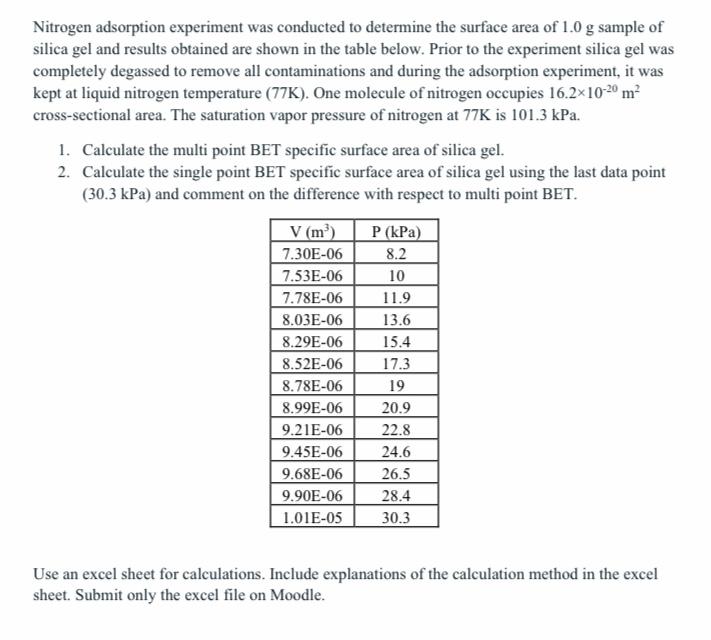
Nitrogen adsorption experiment was conducted to determine the surface area of 1.0g sample of silica gel and results obtained are shown in the table below. Prior to the experiment silica gel was completely degassed to remove all contaminations and during the adsorption experiment, it was kept at liquid nitrogen temperature (77K). One molecule of nitrogen occupies 16.21020m2 cross-sectional area. The saturation vapor pressure of nitrogen at 77K is 101.3kPa. 1. Calculate the multi point BET specific surface area of silica gel. 2. Calculate the single point BET specific surface area of silica gel using the last data point (30.3kPa) and comment on the difference with respect to multi point BET. Use an excel sheet for calculations. Include explanations of the calculation method in the excel sheet. Submit only the excel file on Moodle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


