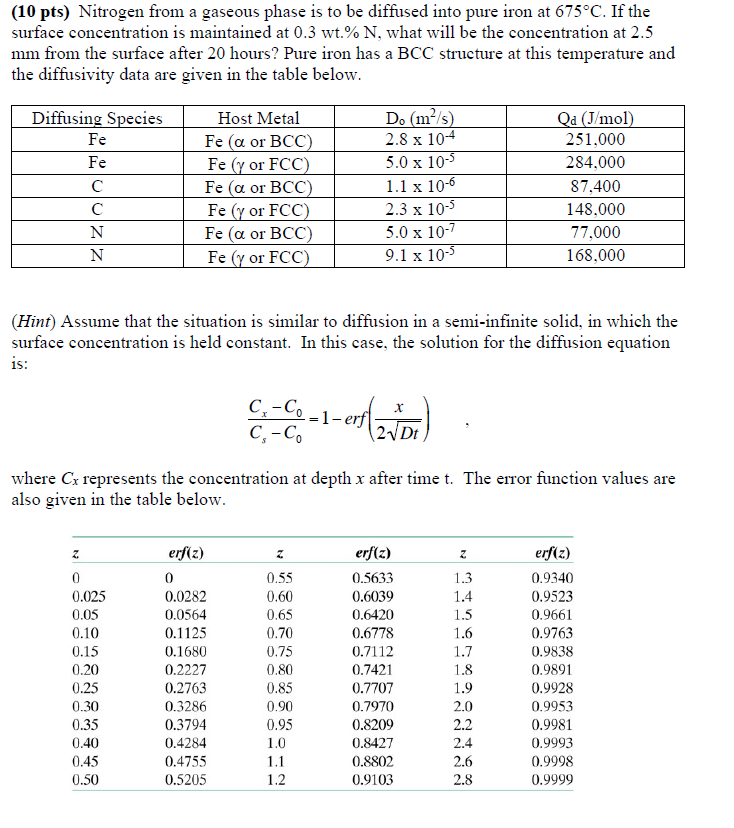
Question: Nitrogen from a gaseous phase is to be diffused into pure iron at 6 7 5 C . If the surface concentration is maintained at
Nitrogen from a gaseous phase is to be diffused into pure iron at If the
surface concentration is maintained at what will be the concentration at
from the surface after hours? Pure iron has a BCC structure at this temperature and
the diffusivity data are given in the table below.
Hint Assume that the situation is similar to diffusion in a semiinfinite solid, in which the
surface concentration is held constant. In this case, the solution for the diffusion equation
is:
erf
where represents the concentration at depth after time The error function values are
also given in the table below. PLEASE PROVIDE DETAILED EXPALANTIONS FOR EACH STEP.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


