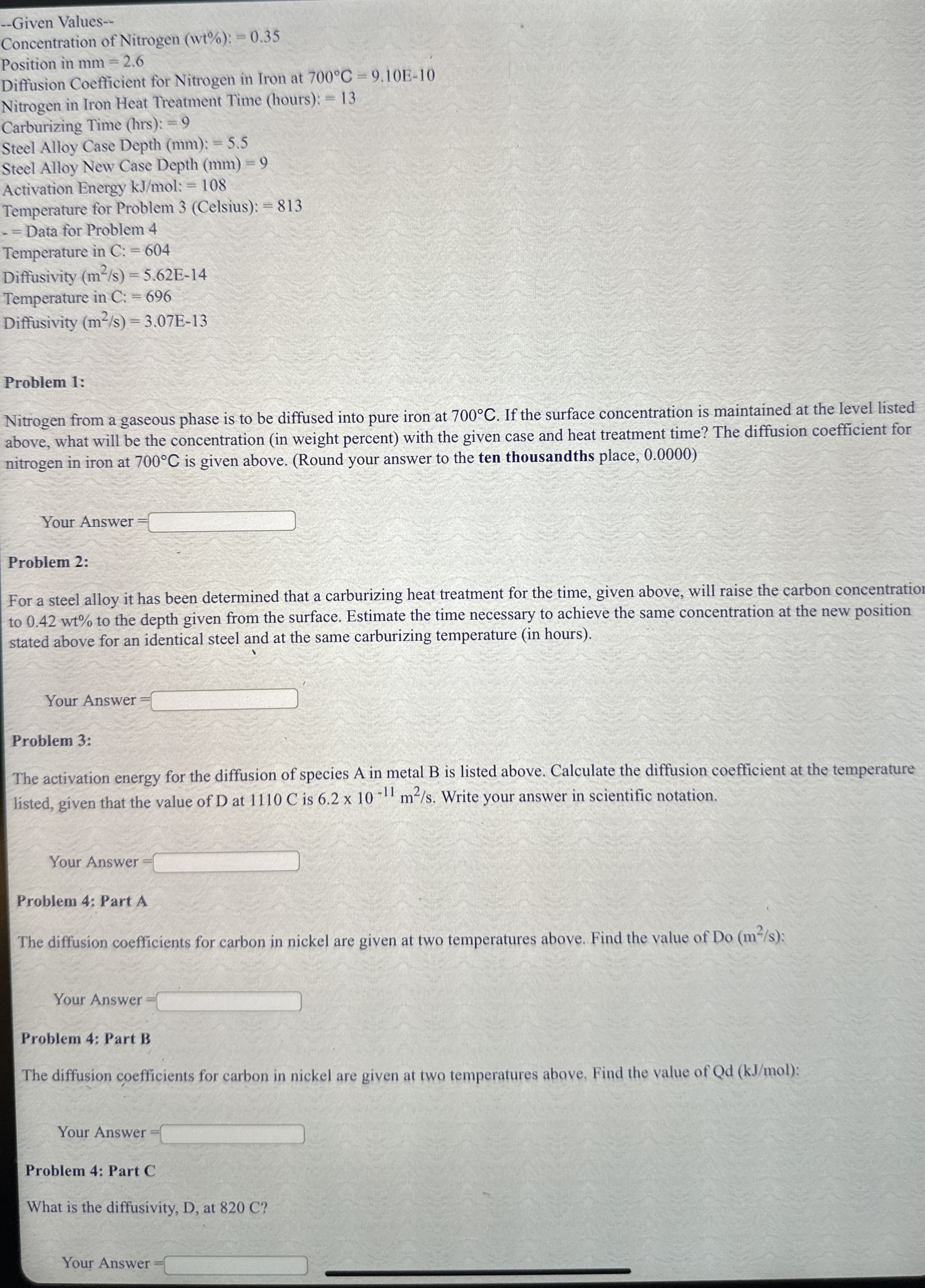
Question: - - Given Values - - Concentration of Nitrogen ( w t % ) : = 0 . 3 5 Position in m m =
Given Values
Concentration of Nitrogen :
Position in
Diffusion Coefficient for Nitrogen in Iron at
Nitrogen in Iron Heat Treatment Time hours :
Carburizing Time hrs:
Steel Alloy Case Depth :
Steel Alloy New Case Depth
Activation Energy kJmol:
Temperature for Problem Celsius :
Data for Problem
Temperature in :
Diffusivity
Temperature in :
Diffusivity
Problem :
Nitrogen from a gaseous phase is to be diffused into pure iron at If the surface concentration is maintained at the level listed
above, what will be the concentration in weight percent with the given case and heat treatment time? The diffusion coefficient for
nitrogen in iron at is given above. Round your answer to the ten thousandths place,
Your Answer
Problem :
For a steel alloy it has been determined that a carburizing heat treatment for the time, given above, will raise the carbon concentration
to to the depth given from the surface. Estimate the time necessary to achieve the same concentration at the new position
stated above for an identical steel and at the same carburizing temperature in hours
Your Answer
Problem :
The activation energy for the diffusion of species A in metal B is listed above. Calculate the diffusion coefficient at the temperature
listed, given that the value of at C is Write your answer in scientific notation.
Your Answer
Problem : Part A
The diffusion coefficients for carbon in nickel are given at two temperatures above. Find the value of @ :
Your Answer
Problem : Part B
The diffusion coefficients for carbon in nickel are given at two temperatures above, Find the value of :
Your Answer
Problem : Part C
What is the diffusivity, D at C
Your Answer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


