Question: Note: Whenever possible type your answers. If you upload any images, each one must have your full name in order to receive credit. This applies

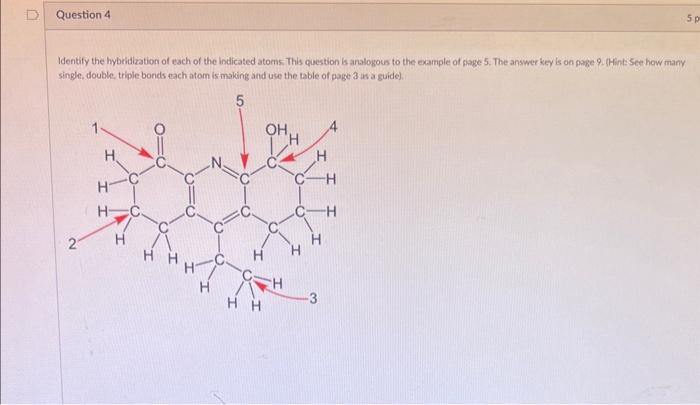


Note: Whenever possible type your answers. If you upload any images, each one must have your full name in order to receive credit. This applies to all assignunents/questions. Indicate why CH3CH2CH2OCH2CH2OH is an oreanic compound but CiClO4 is inorganic. Handdraw (recommended) or use ChemSketch or ChemDraw software to draw the correct structure of a single hydrocarbon that contains both sp? and sp carbans. Label each C atom that is sp 2 and sp. Restrictions: (1) The molecule must contain? carbons. (2) The molecule cannot be such a weird structure or intermediate: no formal charges should be present or implied. Files that are uplaaded sideways or upside down will not be graded. Also, your full name must be present on the image you upload in order to receive credit. Handdraw (recommended) or use ChemSketch or ChemDraw soltware to draw the correct structure of a single molecule that contains an sp - carbon connected to an sp nitrogen. Show all bonds clearly. Label those C and N atoms as sp. Restrictions: (1) The molecule must contain 4 carbons which include a methyl branch. (2) The molecule cannot be such a weird structure or intermediate: no formal charges should be present or inplied. Files that are uploaded sideways or upside down will not be graded. Also. your full name must be present on the image you upiosd in order to receive credit. Identify the hybridiration of each of the indicated atoms. This question is analogous to the example of giage 5. The answer key is on page 9 . thint See how many single, double, tripie bonds each atom is making and use the table of page 3 us a guide]. Indicate the approximate shape (geometry) at each of the 5 atoms indicated in the previous question. Use the table from page 3 as a guide. Insert Fomut fook Tatle Which of the folowing is not true of norane, C4H20 which has a density of 0.79j/mL, melts at - 51C, and beils at 157C ? A) Nonane floats on water. B) Nonane is a lipesid at room temperature Nonane is insoluble in water. D) Nonane is a gas at room temperature. F) More than one correct response: Edit View insent format tools fable Which of the following is a typical property of ionic compounds? A) low boiling points B) high melting points covalent bonding D) non-flammable E) More than one correct response In a line-angle structural formula, each carbon atom is A) clearly written as well as H atoms B) clearly written without any H atoms C) grouped with all other carbon atoms. D) not explicitly shown. E) written in lowercase letters
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


