Question: Now that the order for both reactants is known, the rate constant, k, can be determined. Each of the four trials can be used to
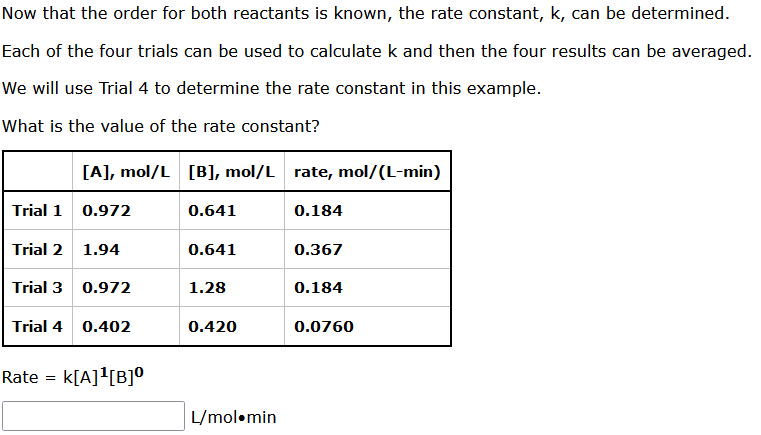
Now that the order for both reactants is known, the rate constant, k, can be determined. Each of the four trials can be used to calculate k and then the four results can be averaged. We will use Trial 4 to determine the rate constant in this example. What is the value of the rate constant? Rate =k[A]1[B]0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


