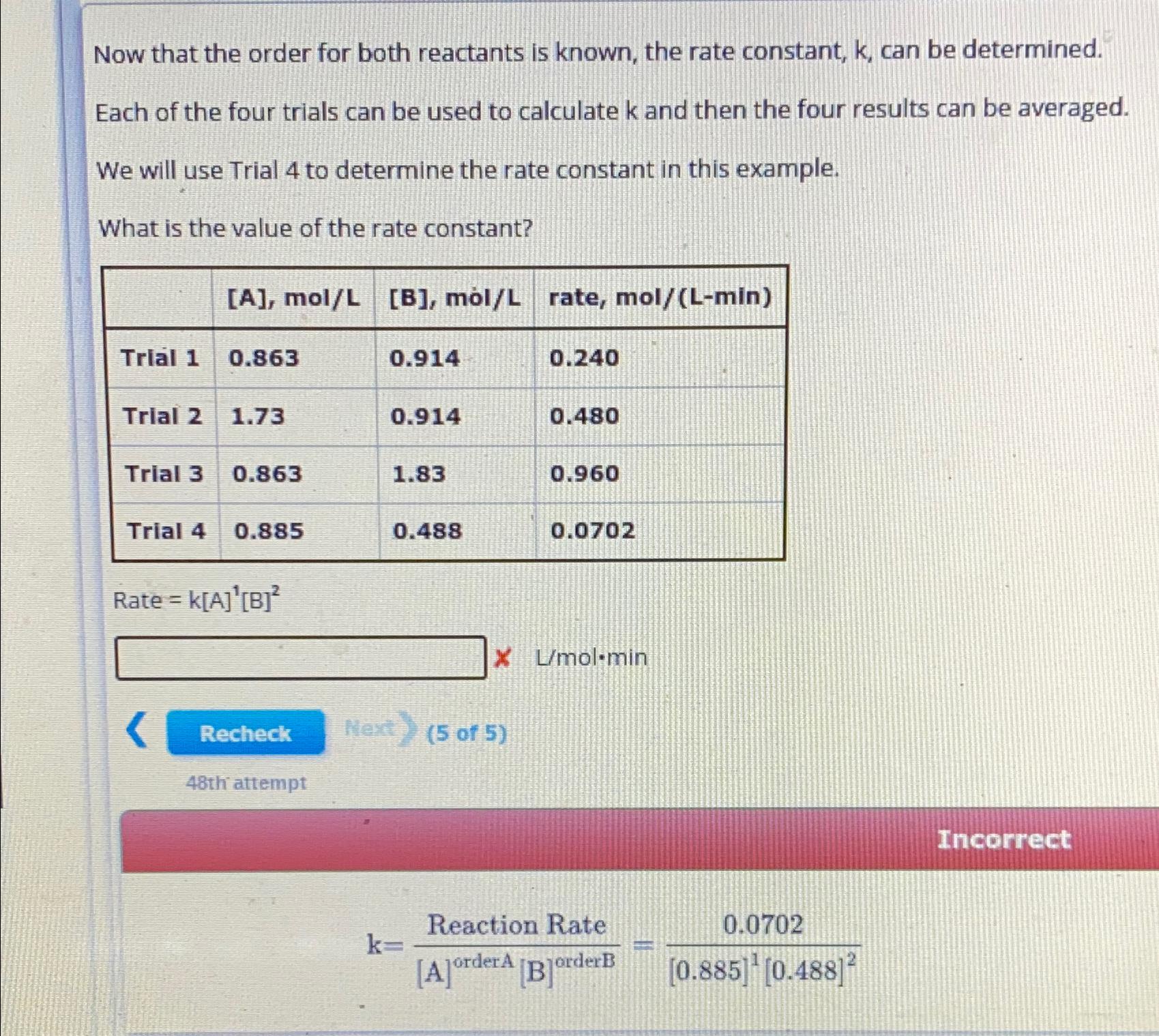
Question: Now that the order for both reactants is known, the rate constant, k , can be determined. Each of the four trials can be used
Now that the order for both reactants is known, the rate constant, k can be determined.
Each of the four trials can be used to calculate and then the four results can be averaged.
We will use Trial to determine the rate constant in this example.
What is the value of the rate constant?
tablerate, molLminTrial Trial Trial Trial
Rate
Lmolmin
of
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


