Question: O Macmillan Learning The element carbon has two naturally occurring isotopes. The isotopic masses and abundances of these isotopes are shown in the table.
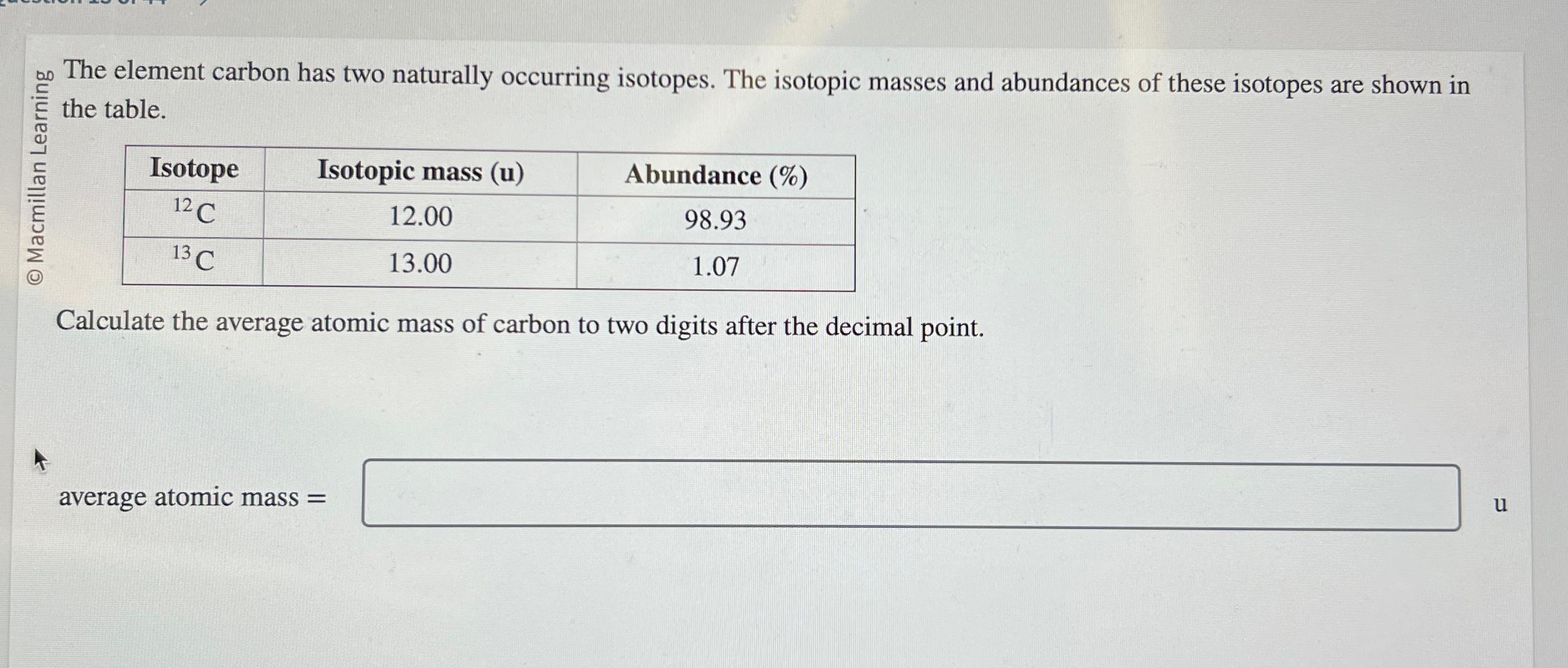
O Macmillan Learning The element carbon has two naturally occurring isotopes. The isotopic masses and abundances of these isotopes are shown in the table. Isotope Isotopic mass (u) 12 C 12.00 13 C 13.00 Calculate the average atomic mass of carbon to two digits after the decimal point. average atomic mass= Abundance (%) 98.93 1.07
Step by Step Solution
3.47 Rating (150 Votes )
There are 3 Steps involved in it
Average Atomic Mass of Carbon Carbon has two isotopes C ... View full answer

Get step-by-step solutions from verified subject matter experts


