Question: Okay so basically my teacher gave us a lab report telling us what we where going to do. So he said to add the topic
Okay so basically my teacher gave us a lab report telling us what we where going to do. So he said to add the topic which is measurement of length, mass, volume and density. Then write the objective, a theory which should include the formulas which are in the lab under measuring the volume. And then materials which are talked about in the lab report it includes the vernier caliper, meter stick, micrometer and electrical scale. Then the procedure of how items are measured and then the result/data. Then calculation he said to include the L1,L2,L3,L4 measurements , and the conclusion and then questions at the end. I already answered the data part you just have to contribute it Into the lab report.I need help turning this into a lab report! Thank you
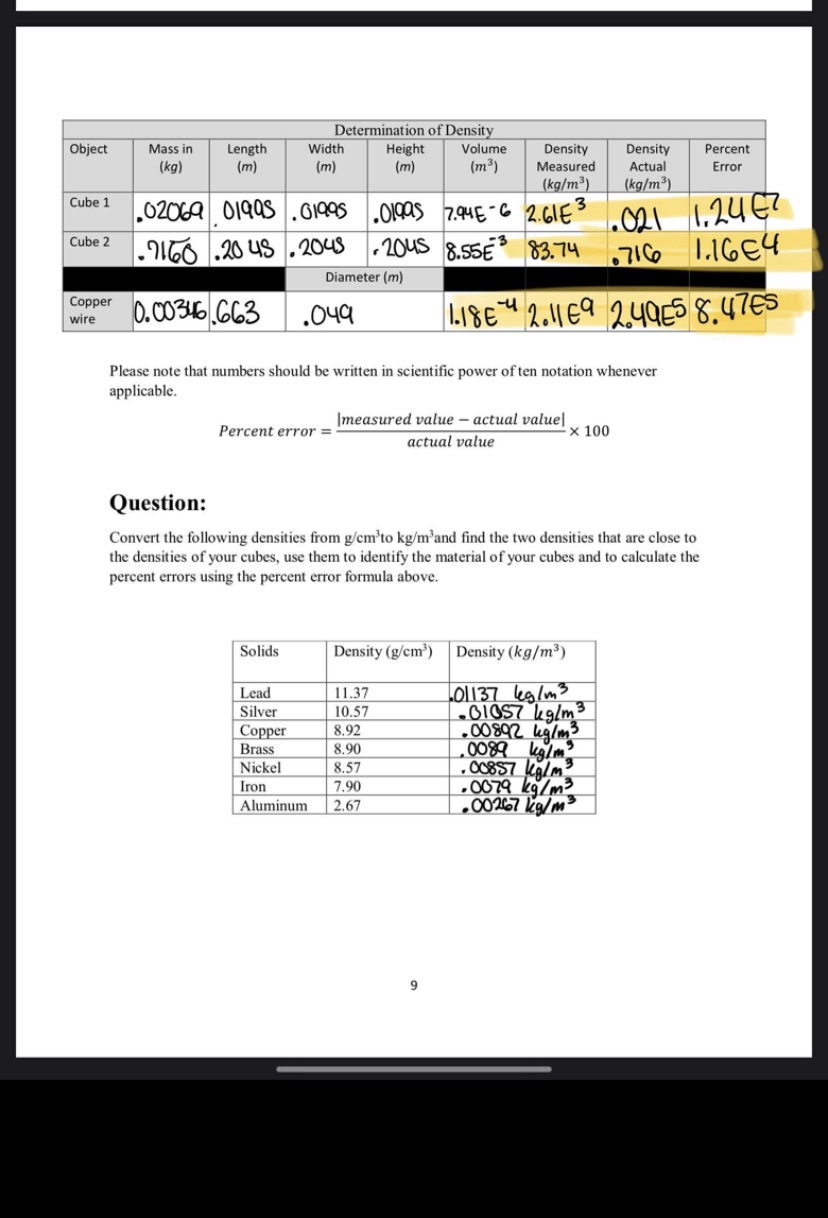
Determination of Density Object Mass in Length Width Height Volume Density Density Percent (kg) (m) (m) (m) (m ) Measured Actual Error (kg/m3) (kg/m3) Cube 1 02069 . Olgas 7.94E - 6 2. GIES 021 1. 24 67 Cube 2 7160 .20 45 . 2048 20us 8.55E 83.74 .716 1.16E4 Diameter (m) Copper wire 10.00346 . 663 . 049 1.18E- 2.11 E9 2.49E5 8. 47 es Please note that numbers should be written in scientific power of ten notation whenever applicable. Percent error = [measured value - actual valuel x 100 actual value Question: Convert the following densities from gem'to kg/m'and find the two densities that are close to the densities of your cubes, use them to identify the material of your cubes and to calculate the percent errors using the percent error formula above. Solids Density (g/cm') Density (kg/m3) Lead 11.37 101 137 leg/m 3 Silver 10.57 Copper 8.92 . 00892 kg/m3 Brass 8.90 0089 big/m Nickel 8.57 . 00857 kalma Iron 7.90 . 0079 kg/m? Aluminum 2.67 . 00267 kg/m3
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


