Question: Old MathJax webview ... fast . Enthalpy of Neutralization data sheet. Mass of inner beaker = 65.12 g. NaOH + HCI NH3-HCI 20.2 20.9 888888
Old MathJax webview
... fast
.
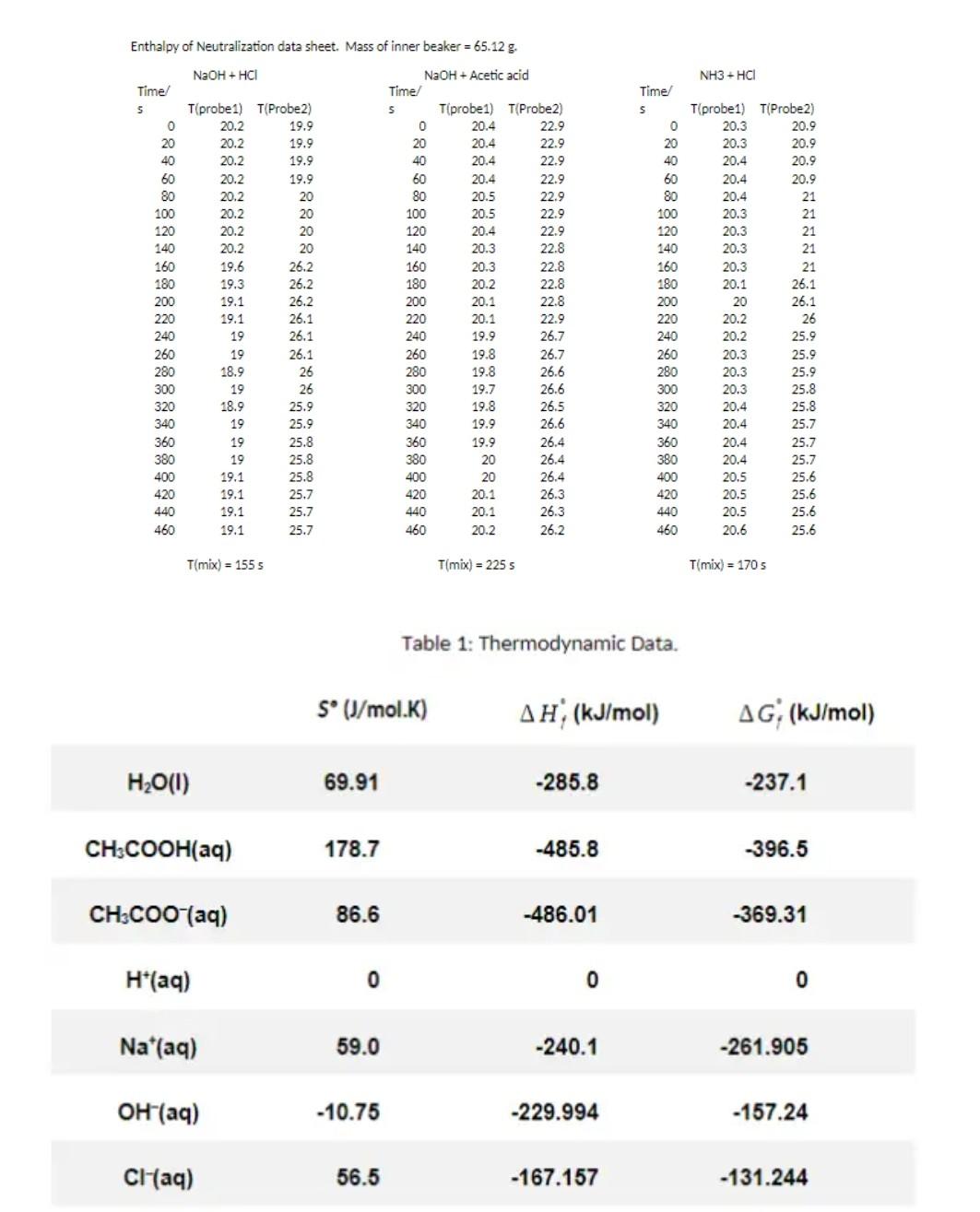
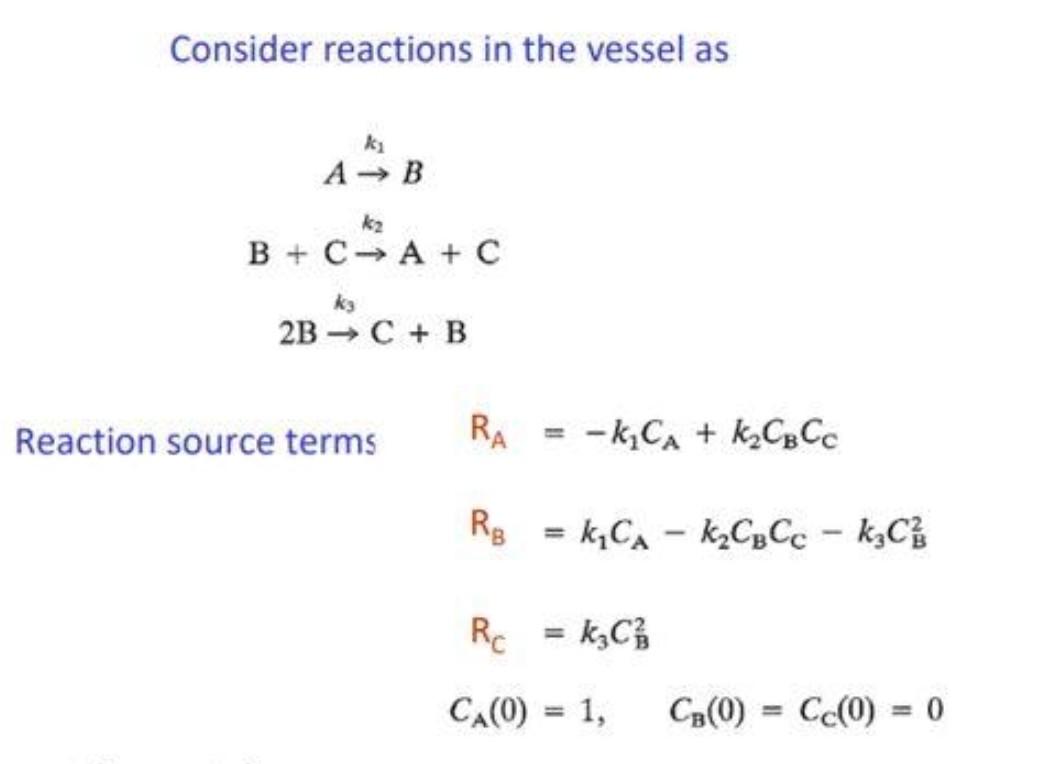
Enthalpy of Neutralization data sheet. Mass of inner beaker = 65.12 g. NaOH + HCI NH3-HCI 20.2 20.9 888888 22.9 Time s 0 20 40 60 80 100 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 460 T(probe1) T(Probe 2) 19.9 20.2 19.9 20.2 19.9 20.2 19.9 20.2 20 20.2 20 20.2 20 20.2 20 19.6 26.2 19.3 26.2 19.1 26.2 19.1 26.1 19 26.1 19 26.1 18.9 26 19 26 18.9 25.9 19 25.9 19 25.8 19 25.8 19.1 25.8 19.1 25.7 19.1 25.7 19.1 25.7 NaOH + Acetic acid Time 5 Tprobe1) T(Probe 2) 0 20.4 22.9 20 20.4 22.9 40 20.4 22.9 60 20.4 22.9 80 20.5 22.9 100 20.5 120 20.4 22.9 140 20.3 22.8 160 20.3 22.8 180 20.2 22.8 200 20.1 22.8 220 20.1 22.9 240 19.9 26.7 260 19.8 280 19.8 26.6 300 19.7 26.6 320 19.8 340 19.9 26.6 360 19.9 26.4 380 20 26.4 400 20 26.4 420 20.1 26.3 440 20.1 26.3 460 20.2 26.2 Time s 0 20 40 60 80 100 120 140 160 180 200 220 240 260 280 300 320 340 360 380 400 420 440 460 T(probe1) T(Probe 2) 20.3 20.3 20.9 20.4 20.9 20.4 20.9 20.4 21 20.3 21 20.3 21 20.3 21 20.3 21 20.1 26.1 20 26.1 20.2 26 20.2 25.9 20.3 20.3 25.9 20.3 25.8 20.4 25.8 20.4 25.7 20.4 25.7 20.4 25.7 20.5 25.6 20.5 25.6 20.5 25.6 20.6 25.6 26.7 25.9 26.5 T(mix) = 155 s T(mix) = 225 Tmix) = 170 5 Table 1: Thermodynamic Data. S* (J/mol.K) , (kJ/mol) AG; (kJ/mol) H2O(1) 69.91 -285.8 -237.1 CH3COOH(aq) 178.7 -485.8 -396.5 CH3COOH(aq) 86.6 -486.01 -369.31 H*(aq) 0 0 0 Na*(aq) 59.0 -240.1 -261.905 OH(aq) -10.75 -229.994 -157.24 CHaq) 56.5 -167.157 -131.244 Consider reactions in the vessel as ki A B k2 B + C A+C ky 2B C + B Reaction source terms RA -k, CA + K C Cc Rg = k C - k CgCc - kzC} Rc = kz C/ CA(0) = 1, C(O) = C(O) = 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


