Question: On most planets, when silicon reacts with even small amounts of oxygen it forms silicates, such as the mineral, quartz, which is made up
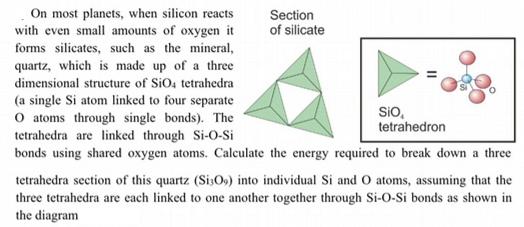
On most planets, when silicon reacts with even small amounts of oxygen it forms silicates, such as the mineral, quartz, which is made up of a three dimensional structure of SiO4 tetrahedra (a single Si atom linked to four separate O atoms through single bonds). The tetrahedra are linked through Si-O-Si bonds using shared oxygen atoms. Calculate the energy required to break down a three tetrahedra section of this quartz (Si309) into individual Si and O atoms, assuming that the three tetrahedra are each linked to one another together through Si-O-Si bonds as shown in the diagram SiO, tetrahedron Section of silicate
Step by Step Solution
There are 3 Steps involved in it
We know that three unit of Sion used to form 6 of S1... View full answer

Get step-by-step solutions from verified subject matter experts


