Question: Only do the 3rd question (according to the question 1). Please show all the stages of the solution in detail. (The specific heat ratio is
Only do the 3rd question (according to the question 1). Please show all the stages of the solution in detail. (The specific heat ratio is = 1.66 for an ideal monoatomic gas and = 1.4 for air, which is predominantly a diatomic gas.)
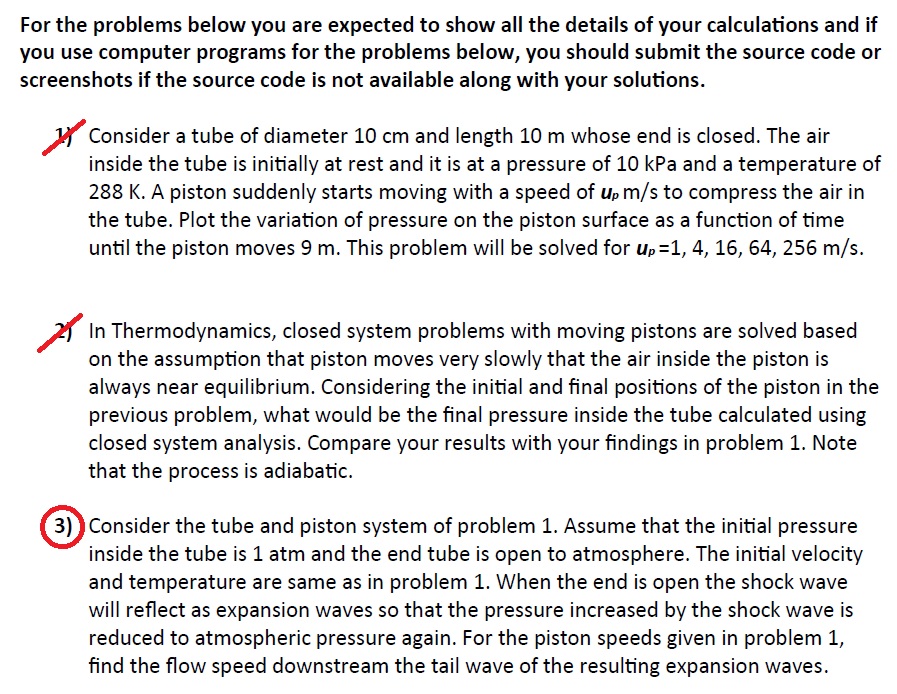
For the problems below you are expected to show all the details of your calculations and if you use computer programs for the problems below, you should submit the source code or screenshots if the source code is not available along with your solutions. 1) Consider a tube of diameter 10cm and length 10m whose end is closed. The air inside the tube is initially at rest and it is at a pressure of 10kPa and a temperature of 288K. A piston suddenly starts moving with a speed of upm/s to compress the air in the tube. Plot the variation of pressure on the piston surface as a function of time until the piston moves 9m. This problem will be solved for up=1,4,16,64,256m/s. 2) In Thermodynamics, closed system problems with moving pistons are solved based on the assumption that piston moves very slowly that the air inside the piston is always near equilibrium. Considering the initial and final positions of the piston in the previous problem, what would be the final pressure inside the tube calculated using closed system analysis. Compare your results with your findings in problem 1 . Note that the process is adiabatic. 3) Consider the tube and piston system of problem 1. Assume that the initial pressure inside the tube is 1atm and the end tube is open to atmosphere. The initial velocity and temperature are same as in problem 1. When the end is open the shock wave will reflect as expansion waves so that the pressure increased by the shock wave is reduced to atmospheric pressure again. For the piston speeds given in problem 1 , find the flow speed downstream the tail wave of the resulting expansion waves
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


