Question: only need help on the ted highlighted second question Calculate the pH of a solution prepared by mixing 0.0860mol of chloroacetic acid plus 0.0170mol of
 only need help on the ted highlighted second question
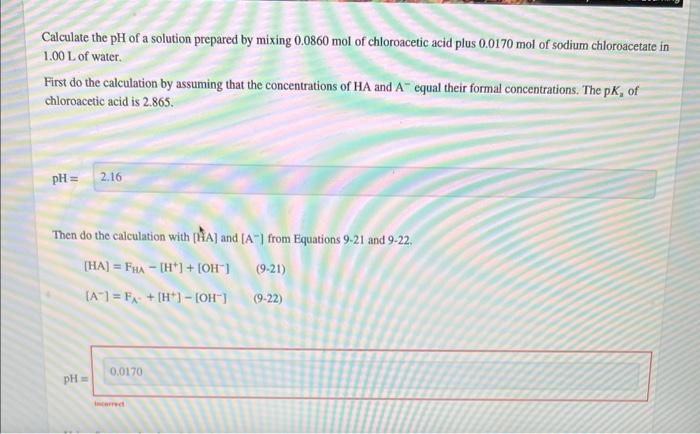
only need help on the ted highlighted second questionCalculate the pH of a solution prepared by mixing 0.0860mol of chloroacetic acid plus 0.0170mol of sodium chloroacetare in 1.00L of water. First do the calculation by assuming that the concentrations of HA and Aequal their formal concentrations. The pKa of chloroacetic acid is 2.865. Then do the calculation with [ttA] and [A]from Equations 921 and 922. [HA]=FHA[H+]+[OH][A]=FA+[H+][OH](921)(922)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


