Question: Only need Task 2 and Task 3 . Sample code provided. Must use Newton-Raphson or bisection method in matlab. The ideal gas equation has been
Only need Task 2 and Task 3. Sample code provided. Must use Newton-Raphson or bisection method in matlab.


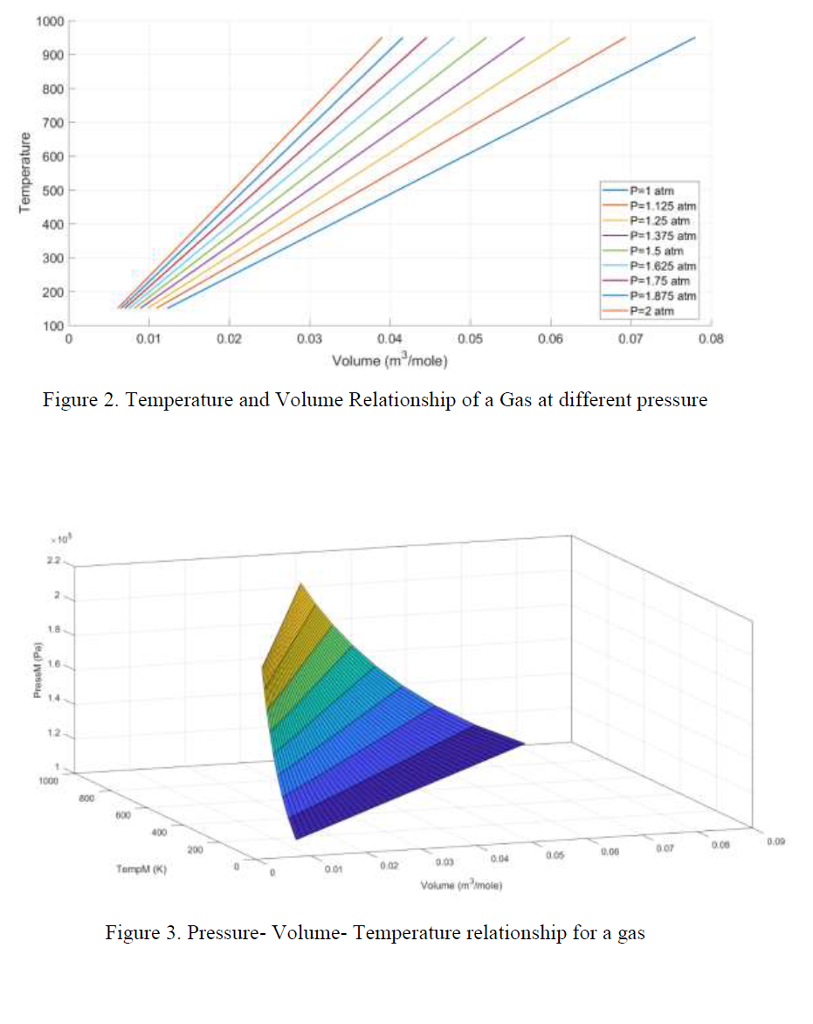
The ideal gas equation has been widely used to relate Temperature, Pressure and Volume of Gases. The equation can be written as RT Where, Vm-Molar volume of gas, R = Gas constant T = Temperature, P = as pressure There are several other equations that characterise gas better than Ideal Gas equation. The Redlich Kwong equation of state is one of them, which is an empirical and algebraic equation. It is generally more accurate than the van der Waals equation and the ideal gas equation at temperatures above the critical temperature. The relation can be written as RT Where 2m 2.5 a 0.42748 c RT, b0.08664 Where, Tc- Critical Temperature, PCritical Pressure Objective of the Project 1. Use Matlab to study Pressure, Volume and Temperature relationship of a Gas of your choice using Redlich-Kwong equation 2. Construct graphical representation of Pressure Volume Relationship using Redlich-Kwong equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


