Question: Open Switch Closed Switch Voltage Source Voltage Source Anode Cathode (Na+) CI- (Na+) Na CI- (Na+) CI CI- (Nat) CI) (Nat) (Na+) (Na+) Na CI-
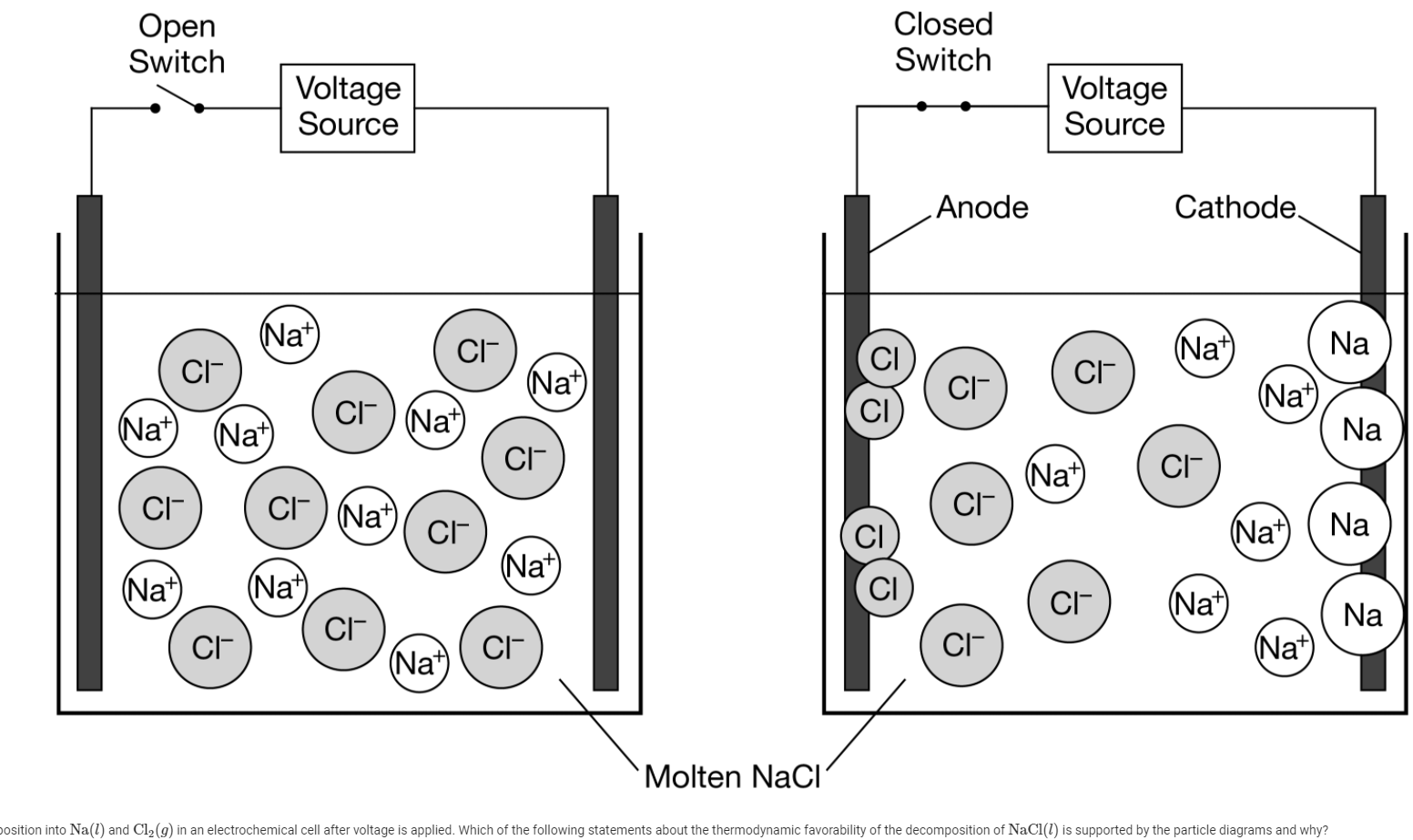

Open Switch Closed Switch Voltage Source Voltage Source Anode Cathode (Na+) CI- (Na+) Na CI- (Na+) CI CI- (Nat) CI) (Nat) (Na+) (Na+) Na CI- (Na+) CI- CI- CI-) Nat) Nat) ( cr CI- CI (Na+) Na (Na+ (Nat (Nat) CI (Na+) CI- Na CI CI- (Na+) (Na+) Molten Naci position into Nal) and C12 (9) in an electrochemical cell after voltage is applied. Which of the following statements about the thermodynamic favorability of the decomposition of NaCl(l) is supported by the particle diagrams and why? The particle diagrams above represent NaCl(l) and its decomposition into Na(l) and C12 (9) in an electrochemical cell after voltage is applied. Which of the following statements about the thermodynamic favorability of the decomposition of NaCl(l) is supported by the particle diagrams and why? A The decomposition is thermodynamically favored because the transfer of electrons to and from the ions occurs at the electrodes. B The decomposition is thermodynamically favored because the formation of a gaseous product results in an increase in entropy. The decomposition is not thermodynamically favored because the pure elements form only after electrical energy is supplied. D The decomposition is not thermodynamically favored because there is a decrease in the number of particles as the reaction proceeds
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


