Question: P8-10B The elementary liquid-phase-series reaction A A kB kc is carried out in a 500-dm3 batch reactor. The initial concentration of A is 1.6 mol/dm3.
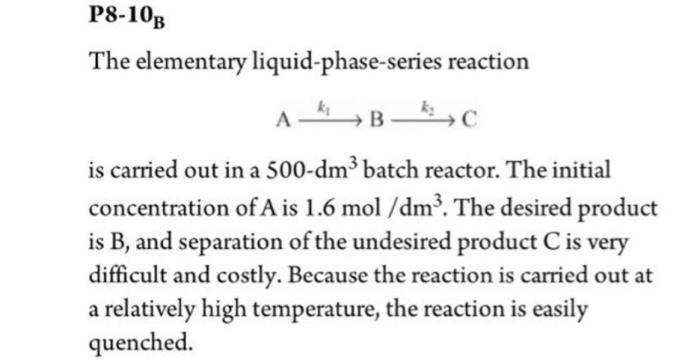
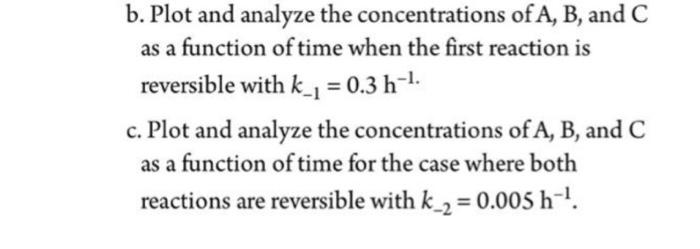
P8-10B The elementary liquid-phase-series reaction A A kB kc is carried out in a 500-dm3 batch reactor. The initial concentration of A is 1.6 mol/dm3. The desired product is B, and separation of the undesired product C is very difficult and costly. Because the reaction is carried out at a relatively high temperature, the reaction is easily quenched. = b. Plot and analyze the concentrations of A, B, and C as a function of time when the first reaction is reversible with k_1 = 0.3 h-1. c. Plot and analyze the concentrations of A, B, and C as a function of time for the case where both reactions are reversible with k_2 = 0.005 h
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


