Question: Part 2: Heat Transfer from Solid to Liquid 1. Place the calorimeter on the balance. Press the tare button to zero the balance, so that
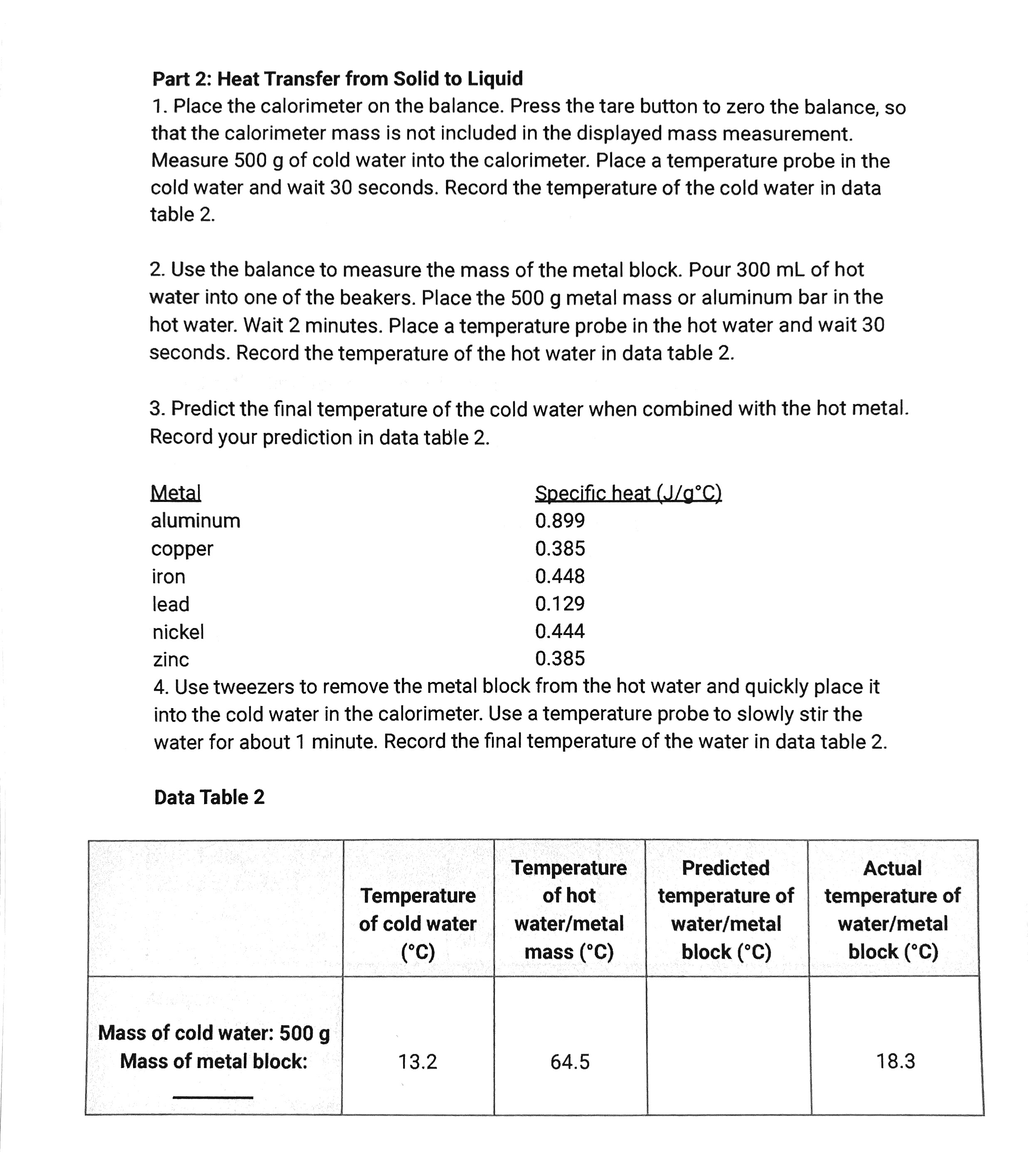
Part 2: Heat Transfer from Solid to Liquid 1. Place the calorimeter on the balance. Press the tare button to zero the balance, so that the calorimeter mass is not included in the displayed mass measurement. Measure 500 g of cold water into the calorimeter. Place a temperature probe in the cold water and wait 30 seconds. Record the temperature of the cold water in data table 2. 2. Use the balance to measure the mass of the metal block. Pour 300 mL of hot water into one of the beakers. Place the 500 9 metal mass or aluminum bar in the hot water. Wait 2 minutes. Place a temperature probe in the hot water and wait 30 seconds. Record the temperature of the hot water in data table 2. 3. Predict the nal temperature of the cold water when combined with the hot metal. Record your prediction in data table 2. Metal Specicheauimm aluminum 0.899 copper 0.385 iron 0.448 lead 0.129 nickel 0.444 zinc 0.385 4. Use tweezers to remove the metal block from the hot water and quickly place it into the cold water in the calorimeter. Use a temperature probe to slowly stir the water for about 1 minute. Record the nal temperature of the water in data table 2. Data Table 2 Temperature Predicted Actual Temperature of hot temperature of temperature of of cold water water/metal water/ metal water/metal mass( C) block( C) block( C) Mass of cold water: 500 9 Mass of metal block
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


