Question: Part 2 X Be sure to review Section 5 . 3 . How can you use the density of iron to find the concentration of
Part
X Be sure to review Section How can you use the density of iron to find the concentration of hydrogen in the alloy?
Review the previous step. Check your mathematics.
If you are having difficulty with this problem, you may wish to review:
Solving Equations
Equations, Symbols, and Units
Powers and Roots
a What is the concentration of hydrogen at the face in kilograms of H per cubic meter?
b What is the concentration of hydrogen at the A face in kilograms of H per cubic meter?
Part
What is the concentration gradient in
eTextbook and Media Part
Calculate the diffusion coefficient, in at the specified temperature.
eTextbook and Media
Attempts: of usedPart
Compute the diffusion flux in s
eTextbook and Media
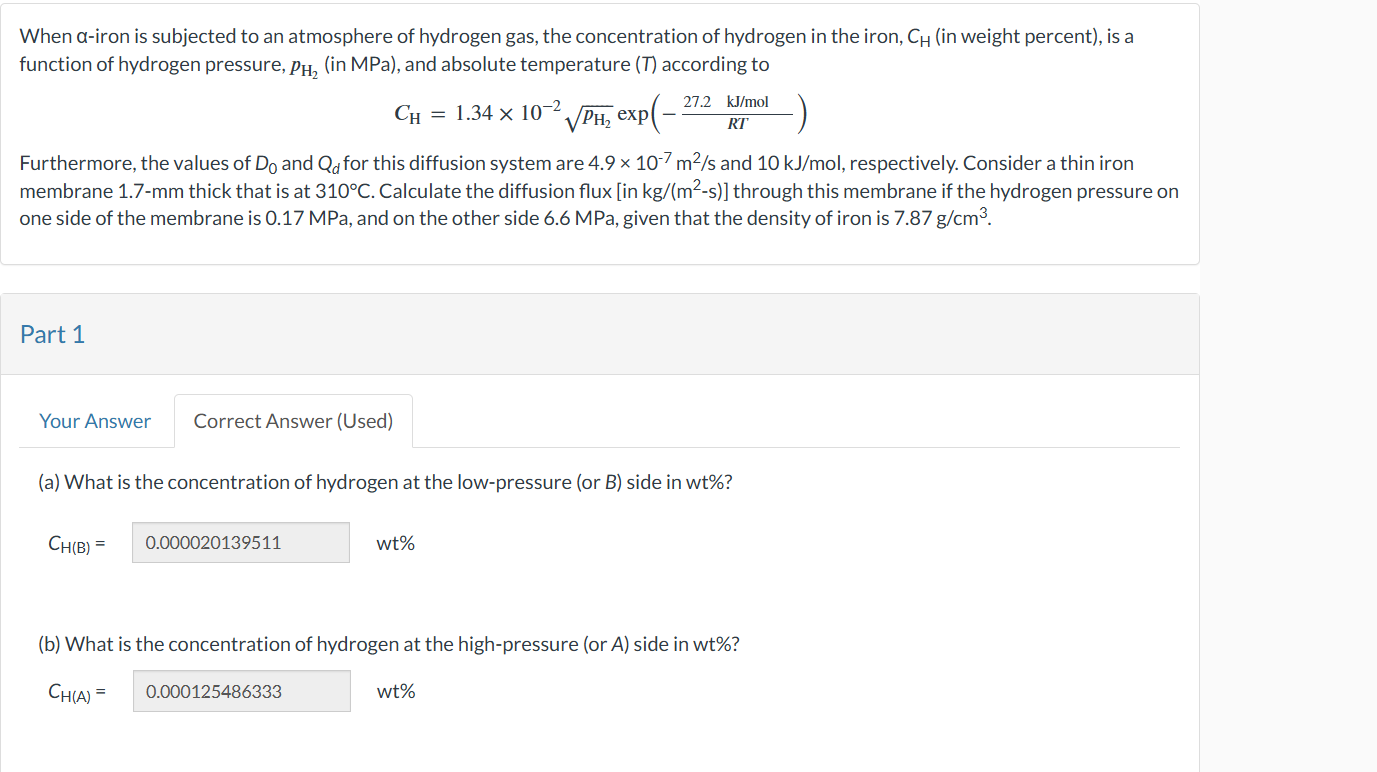
Attempts: of usedWhen iron is subjected to an atmosphere of hydrogen gas, the concentration of hydrogen in the iron, in weight percent is a
function of hydrogen pressure, in MPa and absolute temperature according to
exp
Furthermore, the values of and for this diffusion system are and respectively. Consider a thin iron
membrane thick that is at Calculate the diffusion flux in : through this membrane if the hydrogen pressure on
one side of the membrane is MPa and on the other side MPa given that the density of iron is
Part
Correct Answer Used
a What is the concentration of hydrogen at the lowpressure or B side in wt
b What is the concentration of hydrogen at the highpressure or side in
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


