Question: Part A By what factor is it more likely to find the electron in the ground state of hydrogen at the Bohr radius (To )
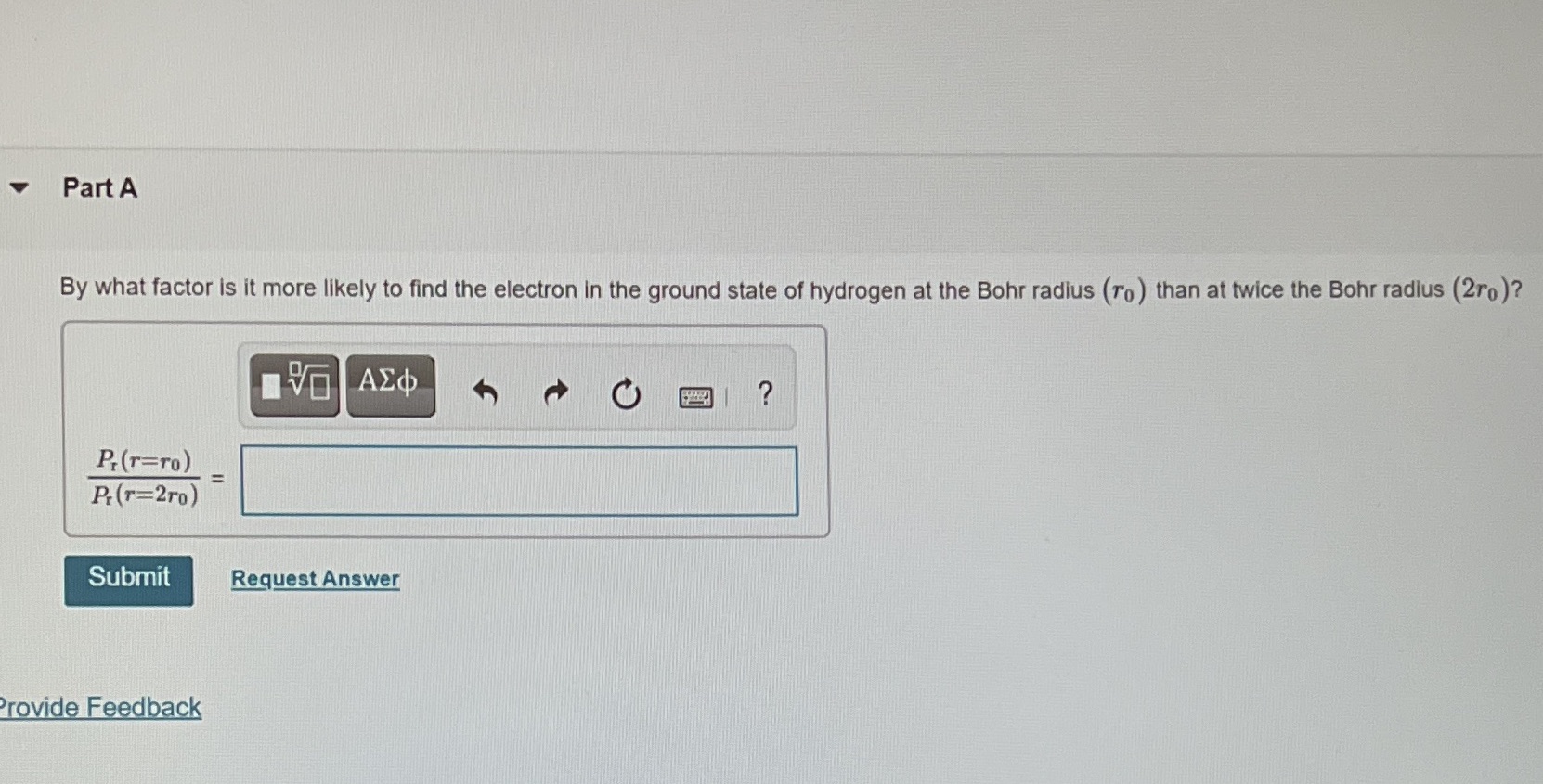
Part A By what factor is it more likely to find the electron in the ground state of hydrogen at the Bohr radius (To ) than at twice the Bohr radius (2ro)? AEd ? P.(T=TO) PI(T=2ro) Submit Request Answer Provide Feedback
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


