Question: 1. You have an electron in a two-dimensional quantum box that is 8.43 nm wide and 8.43 nm. Calculate the energies of the lowest 3
1. You have an electron in a two-dimensional quantum box that is 8.43 nm wide and 8.43 nm. Calculate the energies of the lowest 3 states, and write down their wavefunctions.
2. The Bohr radius is given by ?0 h2 / [? e2 me]. Look up the constants and calculate the value of this expression.
- Starting from the units of the constants you used, show that your Bohr radius has units of meters.
3. Show that the g(?) we guessed in Lecture #32 is indeed a solution to the ? part of the Schroedinger equation for the hydrogen atom.
4. Calculate the mean value of r for an electron in the ground state of a hydrogen atom. The mean value is given by
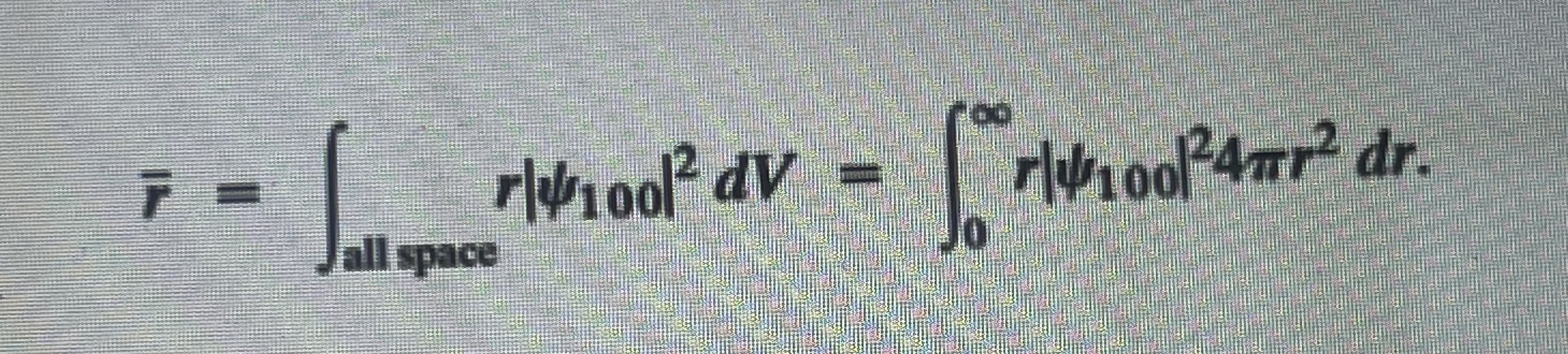
"14100/ dV - 14/100/4mr dr
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


