Question: Part A Currently, CO is being studied as a source of cation atoms for synthesizing organic compounds One possible reaction noves the conversion of Co,
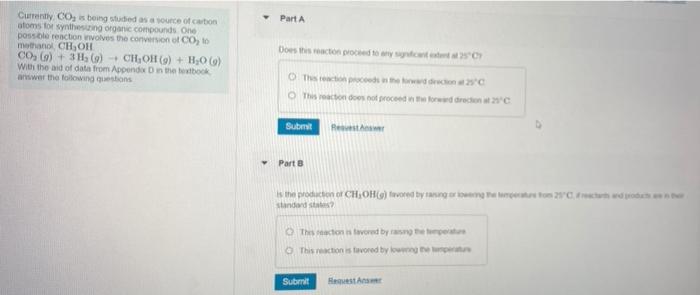
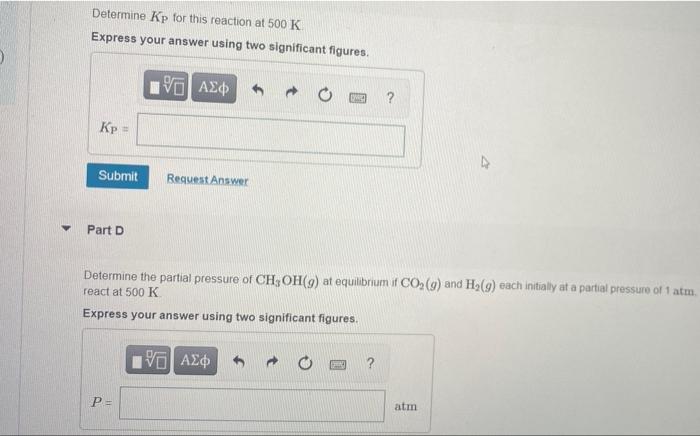
Part A Currently, CO is being studied as a source of cation atoms for synthesizing organic compounds One possible reaction noves the conversion of Co, to methanol CH,OH CO, G) + 3H2() + CHOR() + H206) With the aid of data from Appende in the stock awwer the following questions Does this action proceed to any This proceso con This reaction does not proceed word directen 2C Submit Part is the production of CH,OH() ford byggton 2 w standards The coactions tovored by some This reaction is tevored by low Submit Het Determine Kp for this reaction at 500 K Express your answer using two significant figures. 190 Ad ? Submit Request Answer Part D Determine the partial pressure of CH, OH(9) at equilibrium if CO,(9) and Hz(9) each initially at a partial pressure of 1 atm. react at 500 K Express your answer using two significant figures. | PE atm Part A Currently, CO is being studied as a source of cation atoms for synthesizing organic compounds One possible reaction noves the conversion of Co, to methanol CH,OH CO, G) + 3H2() + CHOR() + H206) With the aid of data from Appende in the stock awwer the following questions Does this action proceed to any This proceso con This reaction does not proceed word directen 2C Submit Part is the production of CH,OH() ford byggton 2 w standards The coactions tovored by some This reaction is tevored by low Submit Het Determine Kp for this reaction at 500 K Express your answer using two significant figures. 190 Ad ? Submit Request Answer Part D Determine the partial pressure of CH, OH(9) at equilibrium if CO,(9) and Hz(9) each initially at a partial pressure of 1 atm. react at 500 K Express your answer using two significant figures. | PE atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


