Question: PART B & C standard conditions El = 2.33 V A voltaic cell employs the following redox reaction: 2 Fe3+ (aq) + 3 Mg(s) +2
PART B & C 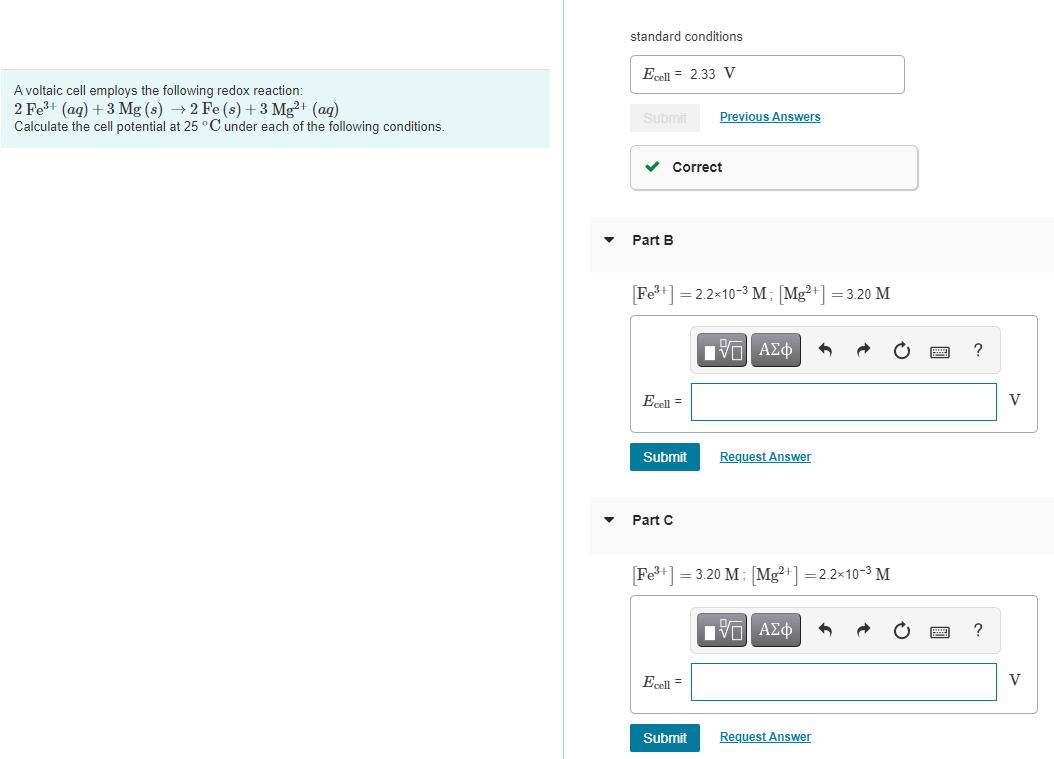
standard conditions El = 2.33 V A voltaic cell employs the following redox reaction: 2 Fe3+ (aq) + 3 Mg(s) +2 Fe (s) + 3 Mg2+ (aq) Calculate the cell potential at 25C under each of the following conditions. Submit Previous Answers Correct Part B Fe3+] =2.2x10-3 M [Mg+] = 3.20 M IVO AEO ? Ecell = V Submit Request Answer Part C Fe3+] = 3.20 M; Mg2+] = 2.2x10-3 M IVO AO Ecell - V Submit Request
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


