Question: What pH is needed to produce this value of Q if the concentration and pressure values are Br2] = 3.00 x 10-4M, Br ]
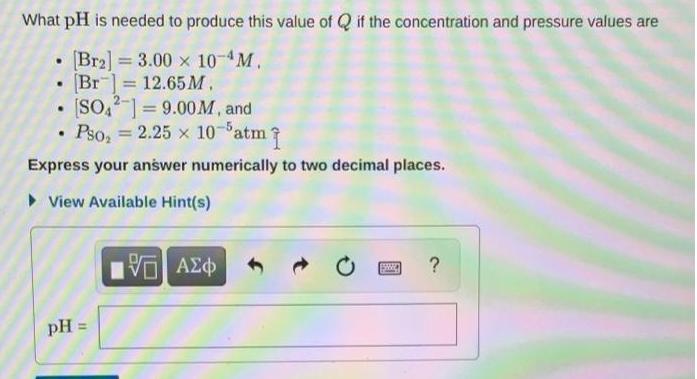
What pH is needed to produce this value of Q if the concentration and pressure values are Br2] = 3.00 x 10-4M, Br ] 12.65M. [SO,-] = 9.00M, and Pso, = 2.25 x 10-Patm %3D %3D Express your answer numerically to two decimal places. > View Available Hint(s) pH =
Step by Step Solution
★★★★★
3.51 Rating (164 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


