Question: Partial and total vapor pressures for methanol (Component B) and water (Component C) solution at 40C is given in the Figure 1 below. a)
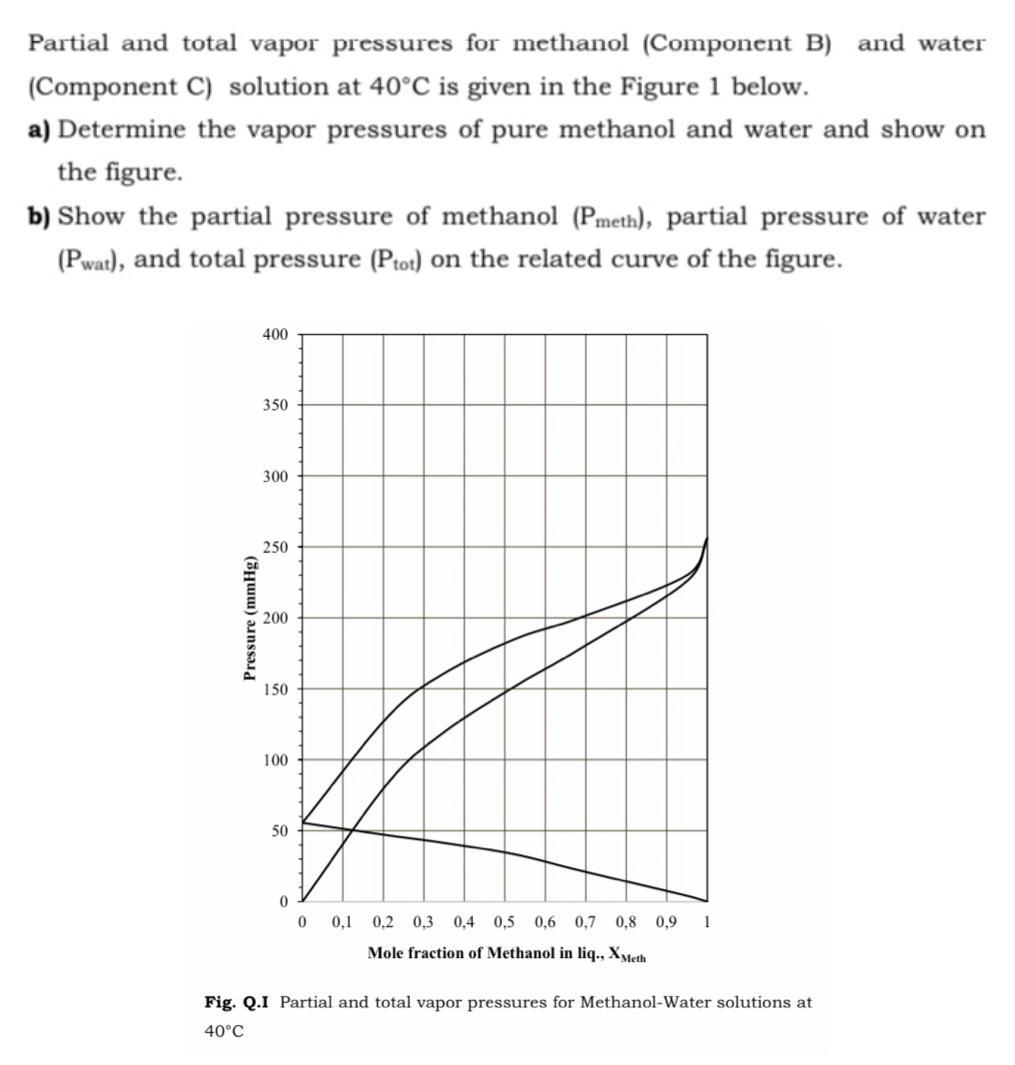
Partial and total vapor pressures for methanol (Component B) and water (Component C) solution at 40C is given in the Figure 1 below. a) Determine the vapor pressures of pure methanol and water and show on the figure. b) Show the partial pressure of methanol (Pmeth), partial pressure of water (Pwat), and total pressure (Ptot) Oon the related curve of the figure. 400 350 300 250 200 150 100 50 0 0,1 0,2 0,3 0,4 0,5 0,6 0,7 0,8 0,9 1 Mole fraction of Methanol in liq., XMeth Fig. Q.I Partial and total vapor pressures for Methanol-Water solutions at 40C Pressure (mmHg)
Step by Step Solution
3.44 Rating (147 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


