Question: Pharmacokinetics (multiple choice and short answer) * Please only someone who knows pharmacokinetics well answer these questions * ALL MINI QUESTIONS BELOW ARE RELATED TO
Pharmacokinetics (multiple choice and short answer)
*Please only someone who knows pharmacokinetics well answer these questions*
ALL MINI QUESTIONS BELOW ARE RELATED TO THE DRUG MIRANDOPRIL
Mirandopril (made up drug name) is a newly marketed small molecule drug (MW = 350) for the treatment of obesity. The company sales representative visits you in
the Pharmacy and provides you with some literature about it.
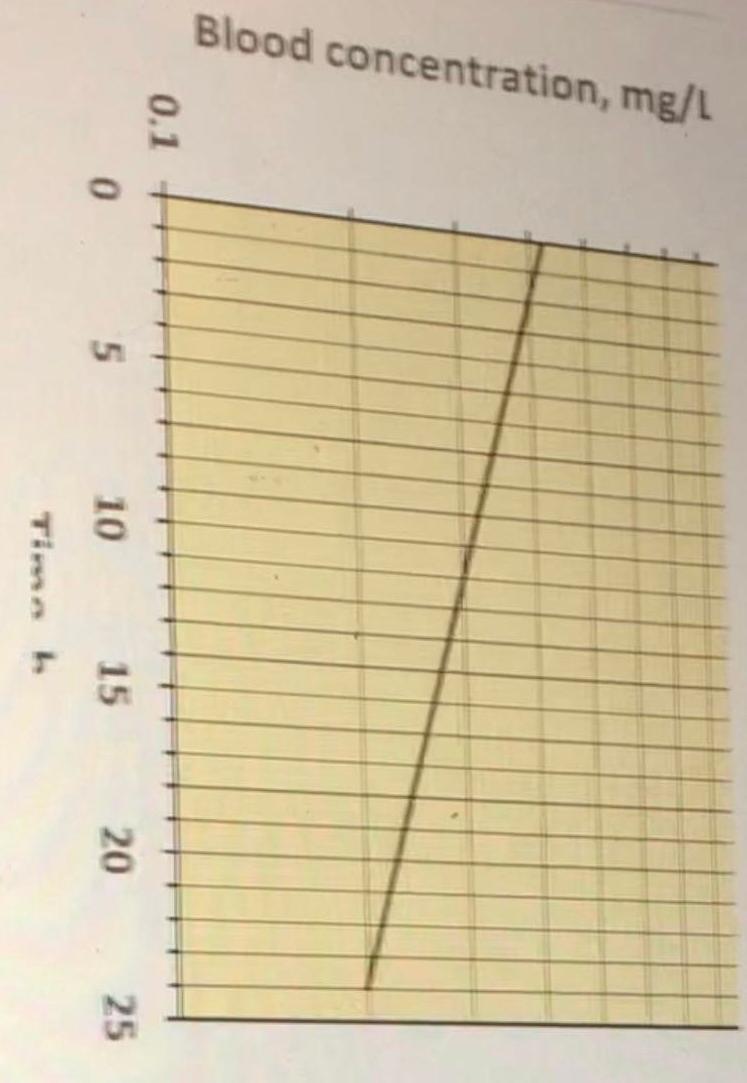
In healthy people, the sales brochure shows the following mean Mirandopril blood concentration vs. time profile after iv doses of 90 mg
The brochure tells you that in healthy people the t1/2 is 22 hours
1) How many compartments does Mirandopril distribute to? (1 pt)
1
2
Cannot tell from the figure provided
2) At what time (in hours) after a 90 mg injection does the mean concentration fall to 0.3 mg/mL?
3) The mean AUC in blood is estimated to be 13.5 mg x h/L after a single 90 mg dose. What is the estimated total body clearance (CL) in L/h? Provide to 3 significant figures. (1 pt)
4) What is the average Vd of Mirandopril in healthy volunteers, in L? Provide to 3 significant figures. (1 pt)
The brochure tells you that 8% of the 90 mg dose was recovered in the urine, with the remainder being metabolized by the liver. The unbound fraction in the blood fluids (same in plasma, serum and whole blood) is 5% in healthy subjects.
Based on this answer the following:
5) What is the renal clearance of Mirandopril, in L/h? Provide to 3 significant figures. (1 pt)
6) What is the hepatic clearance of Mirandopril, in L/h? Provide to 3 significant figures. (1 pt)
In the healthy subjects, the average hepatic flow is 90 L/h.
7) What is the Mirandopril hepatic extraction ratio (E) in healthy volunteers? Provide to 3 significant figures. (1 pt)
8) What is the estimated Clint of Mirandopril, in L/h. Provide to 3 significant figures. (1 pt)
9) What is the mean CL(prime)int of Mirandopril in these subjects, in L/h. (1 pt)
10) The bioavailability of Mirandopril after 90 mg tablets is 78%. What is the GI availability (fg)? Provide to 3 significant figures. (1 pt)
The healthy subjects have a mean creatinine clearance of 120 mL/min (7.2 L/h).
Blood concentrations of Mirandopril are exactly the same as its serum concentrations. Hence, clearances based on blood are the same as clearances based on serum concentration measures.
11) What mechanism(s) can we expect, based on its molecular weight (350 daltons) and other factors given above, to contribute to the renal clearance of Mirandoprils in healthy subjects? (1 pt)
Glomerular filtration
Tubular secretion
Tubular absorption
12) Mirandopril is a weak acid. If procainamide, a weak base excreted into the urine, was given with Mirandopril, what would the effect be on the renal clearance of Mirandopril? (1 pt)
Decrease
No effect
Increase
13) Mirandopril is a weak acid. If sodium bicarbonate was given with Mirandopril, what would the effect be on the renal clearance of Mirandopril? (1 pt)
Decrease
No effect
Increase
14) Which disease state below is likely to lead to a decrease in the unbound fraction (fu) of Mirandopril? (1 pt)
Cirrhosis
Trauma
Chronic renal failure
Acute rheumatoid arthritis
None of the above will decrease the fu
Mirandopril is metabolized by CYP3A4 and when given with ketoconazole, its CL(prime)int will decrease by a factor of two
Oral formulations of Mirandopril come in strengths of 45, 90 and 180 mg
15) If a patient was stable on Mirandopril with 90 mg orally qd, what dose would we likely have to give the patient to attain the same level of effect if ketoconazole was added to the drug therapy? (1pt)
45 mg qd
The same dose (90 mg qd)
180 mg qd
Ketoprofen is a nonsteroidal anti-inflammatory drug known to displace Mirandopril from its plasma protein binding sites (increases fu by a factor of 2).
16) What is the expected Vd in a patient if ketoprofen was also given (assume a blood volume of 5 L)? Use youre answer to question 12 above to help you. Provide to 3 significant figures (1 pt)
17) If a patient was begun on an iv infusion of Mirandopril while taking ketoprofen, what would be the approximate time needed to reach steady-state concentrations (90%), in hours? Provide to 3 significant figures (1 pt).
18) In healthy volunteers, a 90 mg dose of Mirandopril gave an AUC of 13.5 mg x h/L. If an average subject was also given ketoprofen with 90 mg Mirandopril, what would be the approximate AUC of Mirandopril as measured by a standard assay method (total drug concentrations)? (1 pt)
3.4 mg x h/L
6.75 mg x h/L
13.5 mg x h/L
27 mg x h/L
54 mg x h/L
19) In order to obtain the same level of drug effect as 90 mg qd without ketoprofen, what Mirandopril dose should be given in the presence of ketoprofen? (1 pt)
45 mg qd
90 mg qd
180 mg qd
Blood concentration, mg/L
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


