Question: Physics Thermodynamics Test mass. 10. The table below shows data from a heating experiment Involving four substances of equal Heat Substance Change in Added Temperature
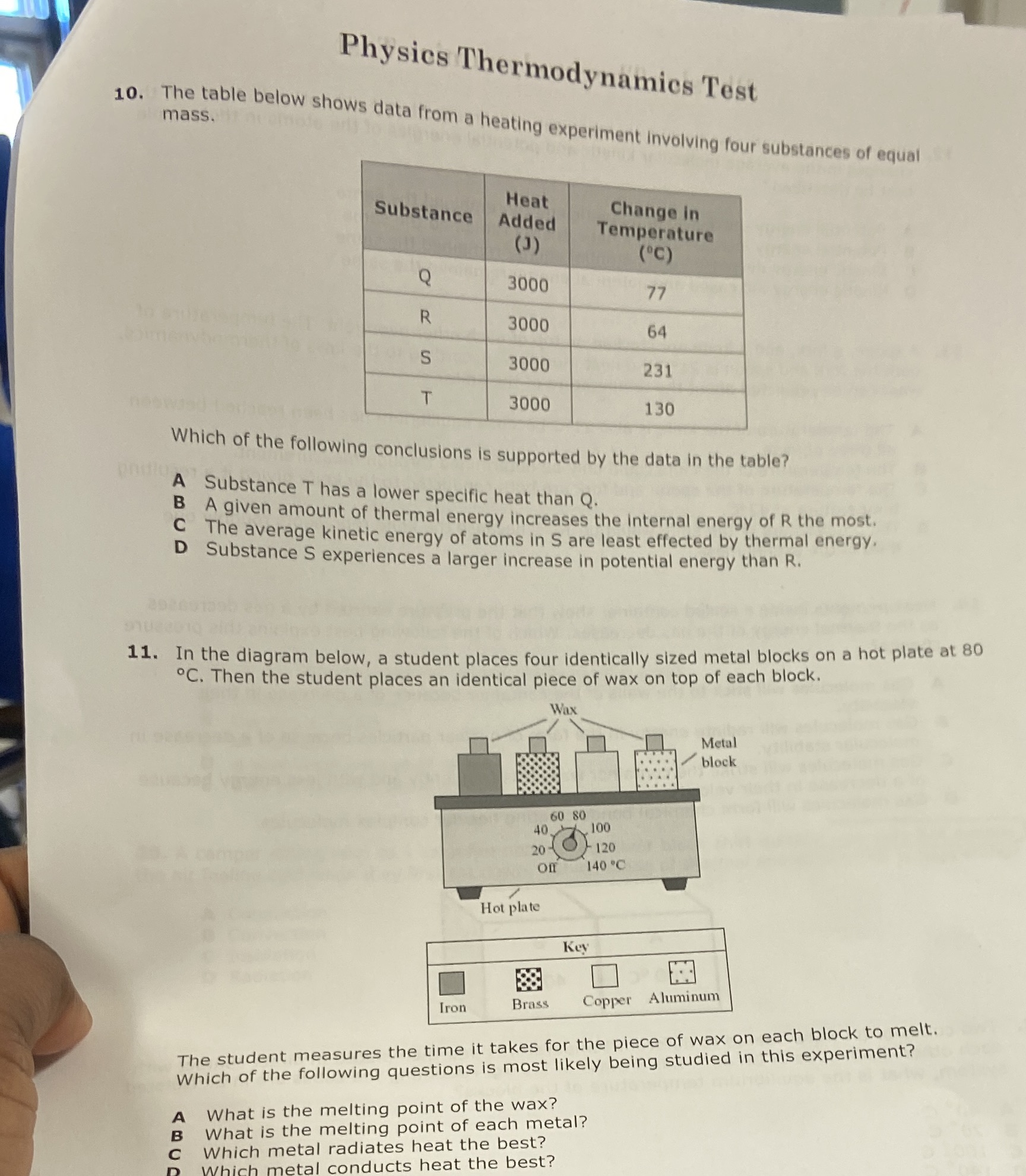
Physics Thermodynamics Test mass. 10. The table below shows data from a heating experiment Involving four substances of equal Heat Substance Change in Added Temperature (J) ('C) Q 3000 77 R 3000 64 S 3000 231 T 3000 130 Which of the following conclusions is supported by the data in the table? A Substance T has a lower specific heat than Q. B A given amount of thermal energy increases the internal energy of R the most. C The average kinetic energy of atoms in S are least effected by thermal energy. D Substance S experiences a larger increase in potential energy than R. 11. In the diagram below, a student places four identically sized metal blocks on a hot plate at 80 .C. Then the student places an identical piece of wax on top of each block. Wax Metal block 60 80 40100 20 0 120 off 140 .C Hot plate Key Brass Copper Aluminum Iron The student measures the time it takes for the piece of wax on each block to melt. Which of the following questions is most likely being studied in this experiment? What is the melting point of the wax? A What is the melting point of each metal? Which metal radiates heat the best? Wh metal conducts heat the best
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


