Question: please answer all (first pic is an example) dissolve in water to give ionic species - Soluble ionic compounds: Example: NaCl(s)Na+(aq)H2O+Cl(aq) - Covalent compounds that
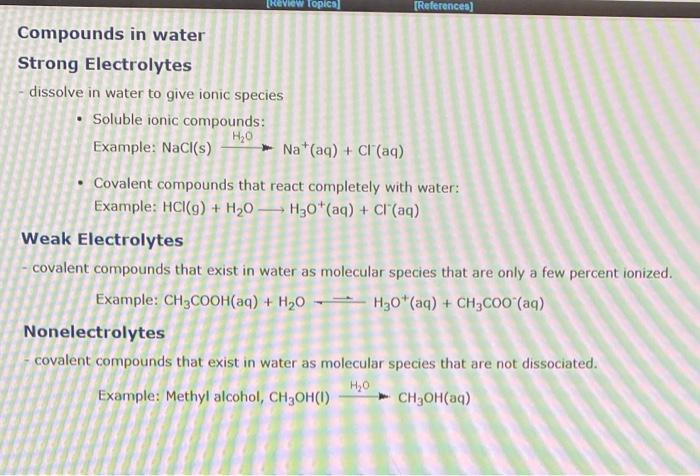

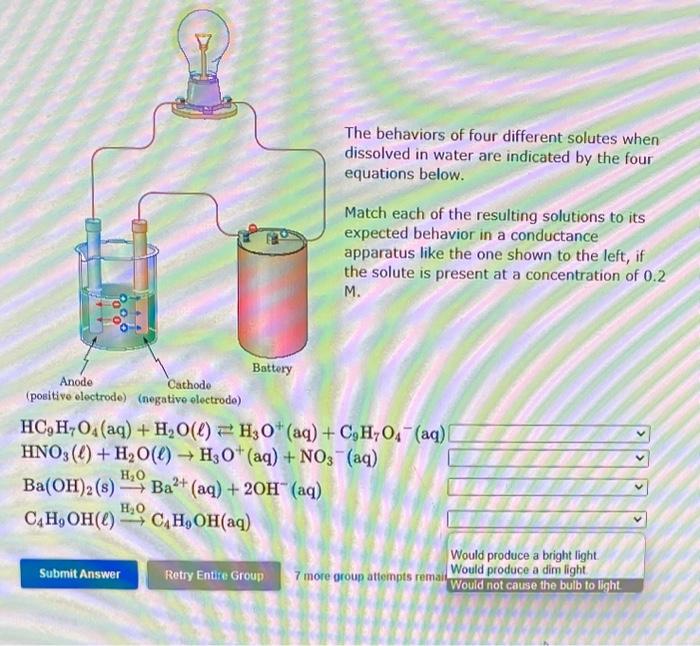

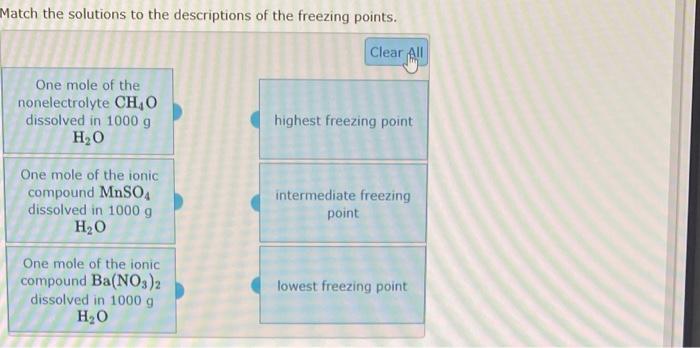
dissolve in water to give ionic species - Soluble ionic compounds: Example: NaCl(s)Na+(aq)H2O+Cl(aq) - Covalent compounds that react completely with water: Example: HCl(g)+H2OH3O+(aq)+Cl(aq) Weak Electrolytes - covalent compounds that exist in water as molecular species that are only a few percent ionized. Example: CH3COOH(aq)+H2OH3O+(aq)+CH3COO(aq) Nonelectrolytes covalent compounds that exist in water as molecular species that are not dissociated. Example: Methyl alcohol, CH3OH(I)CH3OH(aq)H2O Identify each of the following as a strong electrolyte, a weak electrolyte or a nonelectrolyte, based on its behavior when dissolved in water. CaBr2(s)H2OCa2+(aq)+2Br(aq)HOCH2CH2OH()H2OHOCH2CH2OH(aq)HClO(aq)+H2O()H3O+(aq)+ClO(aq)HNO3()+H2O()H3O+(aq)+NO3(aq) 7 more group atf The behaviors of four different solutes when dissolved in water are indicated by the four equations below. Match each of the resulting solutions to its expected behavior in a conductance apparatus like the one shown to the left, if the solute is present at a concentration of 0.2 M. HC9H7O4(aq)+H2O()H3O+(aq)+C9H7O4(aq)HNO3()+H2O()H3O+(aq)+NO3(aq)Ba(OH)2(s)H2OBa2+(aq)+2OH(aq)C4H9OH()H2OC4H9OH(aq) Match the solutions to the descriptions of the freezing points. One mole of the ionic compound NaNO3 dissolved in 1000g H2O One mole of the nonelectrolyte C6H12O6 dissolved in 1000gH2O intermediate freezing point One mole of the ionic compound Pb(NO3)2 dissolved in 1000g lowest freezing point Match the solutions to the descriptions of the freezing points. One mole of the nonelectrolyte CH4O dissolved in 1000g H2O One mole of the ionic compound MnSO4 dissolved in 1000g H2O intermediate freezing point One mole of the ionic compound Ba(NO3)2 dissolved in 1000g H2O
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


