Question: please answer all parts to the question , will rate if correct , thank you ! Reagents a. HX b. HBr,H2O2,hv m. Na/NH3 c. H2O,H2SO4
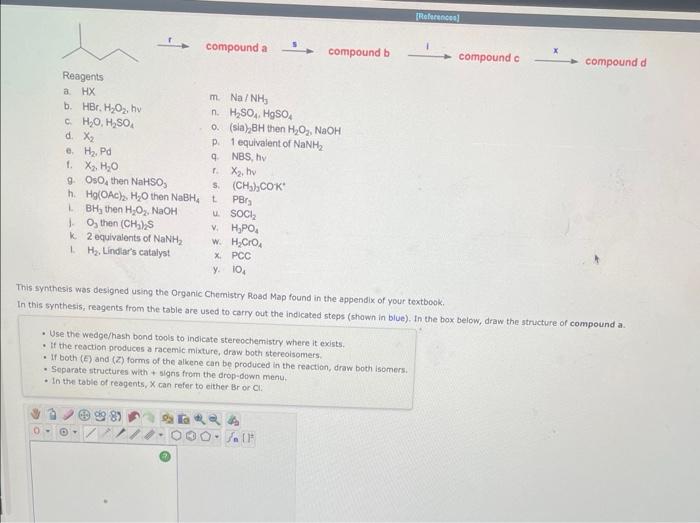
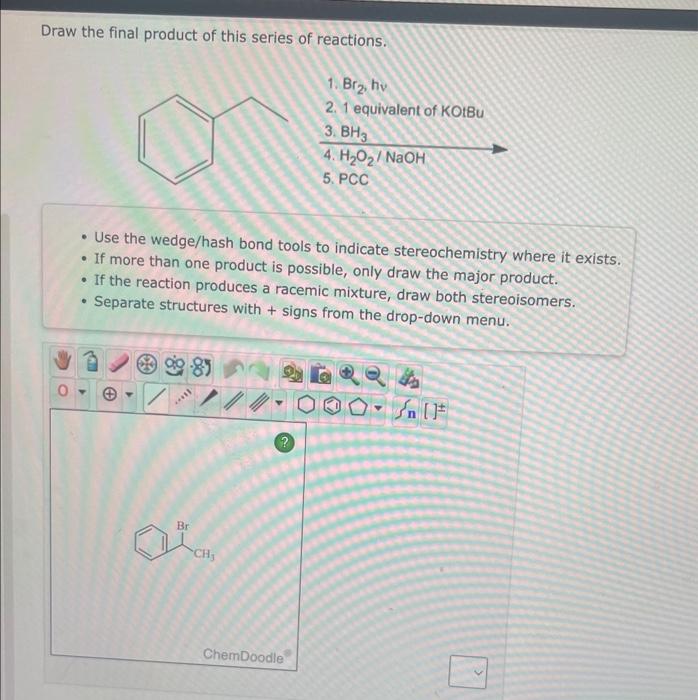
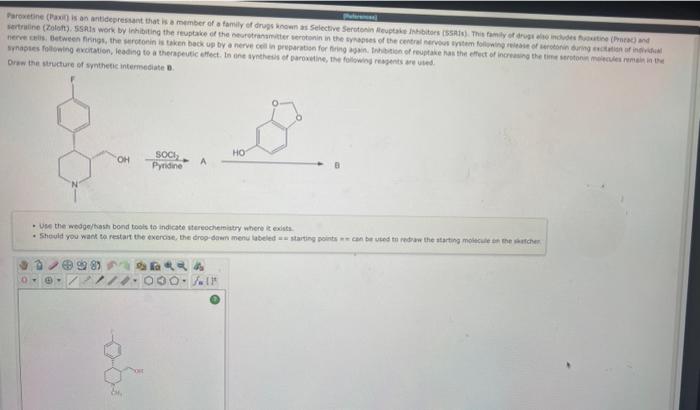
Reagents a. HX b. HBr,H2O2,hv m. Na/NH3 c. H2O,H2SO4 n. H2SO4,HgSO4 d. X2 a. (sia) BH then H2O2,NaOH e. H2,Pd p. 1 equivalent of NaNH2 t. X2,H2O q. NBS, hy r. X2,hv L. BH3 then H2O2.NaOH u SOCl2 1. O3 then (CH2)S v. H3PO4 k. 2 equivalents of NaNH2 w. H2CrO4 1. H2. Lindlar's catalyst x. PCC y. IO4 This synthesis was designed using the Organic Chemistry Road Map found in the appendix of your textbook. In this synthesis, reagents from the table are used to carry out the indicated steps (shown in blue). In the box below, draw the structure of compound a. - Use the wedge/hash bond toots to indicate stereochemistry where it exists. - If the reaction produces a racemic mixture, draw both stereoisomers. - If both () and (z) forms of the alkene can be produced in the reaction, draw both isomers. - Separate structures with + signs from the arop-oown menu. - In the tabie of reapents, X can refer to either Br or Cl. Draw the final product of this series of reactions. 1. Br2,hv 2. 1 equivalent of KOtBu 3. BH3 5. PCC - Use the wedge/hash bond tools to indicate stereochemistry where it exists. - If more than one product is possible, only draw the major product. - If the reaction produces a racemic mixture, draw both stereoisomers. - Separate structures with + signs from the drop-down menu. Orsw the sucture of synthencic intermeciate 0. - UP the medgertiosh bond toois te indicate itareocheinitary where ic exista. Reagents a. HX b. HBr,H2O2,hv m. Na/NH3 c. H2O,H2SO4 n. H2SO4,HgSO4 d. X2 a. (sia) BH then H2O2,NaOH e. H2,Pd p. 1 equivalent of NaNH2 t. X2,H2O q. NBS, hy r. X2,hv L. BH3 then H2O2.NaOH u SOCl2 1. O3 then (CH2)S v. H3PO4 k. 2 equivalents of NaNH2 w. H2CrO4 1. H2. Lindlar's catalyst x. PCC y. IO4 This synthesis was designed using the Organic Chemistry Road Map found in the appendix of your textbook. In this synthesis, reagents from the table are used to carry out the indicated steps (shown in blue). In the box below, draw the structure of compound a. - Use the wedge/hash bond toots to indicate stereochemistry where it exists. - If the reaction produces a racemic mixture, draw both stereoisomers. - If both () and (z) forms of the alkene can be produced in the reaction, draw both isomers. - Separate structures with + signs from the arop-oown menu. - In the tabie of reapents, X can refer to either Br or Cl. Draw the final product of this series of reactions. 1. Br2,hv 2. 1 equivalent of KOtBu 3. BH3 5. PCC - Use the wedge/hash bond tools to indicate stereochemistry where it exists. - If more than one product is possible, only draw the major product. - If the reaction produces a racemic mixture, draw both stereoisomers. - Separate structures with + signs from the drop-down menu. Orsw the sucture of synthencic intermeciate 0. - UP the medgertiosh bond toois te indicate itareocheinitary where ic exista
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


