Question: Please answer all subparts. Struggling with this practice question! - + 2: [6] The combustion of methane (natural gas) is described by the following reaction:
Please answer all subparts. Struggling with this practice question!
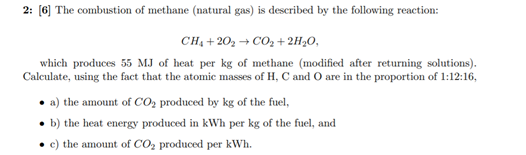
- + 2: [6] The combustion of methane (natural gas) is described by the following reaction: CH. +20, +CO2 +2H20, which produces 55 MJ of heat per kg of methane (modified after returning solutions). Calculate, using the fact that the atomic masses of H, C and O are in the proportion of 1:12:16, a) the amount of CO2 produced by kg of the fuel, b) the heat energy produced in kWh per kg of the fuel, and c) the amount of CO2 produced per kWh
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


