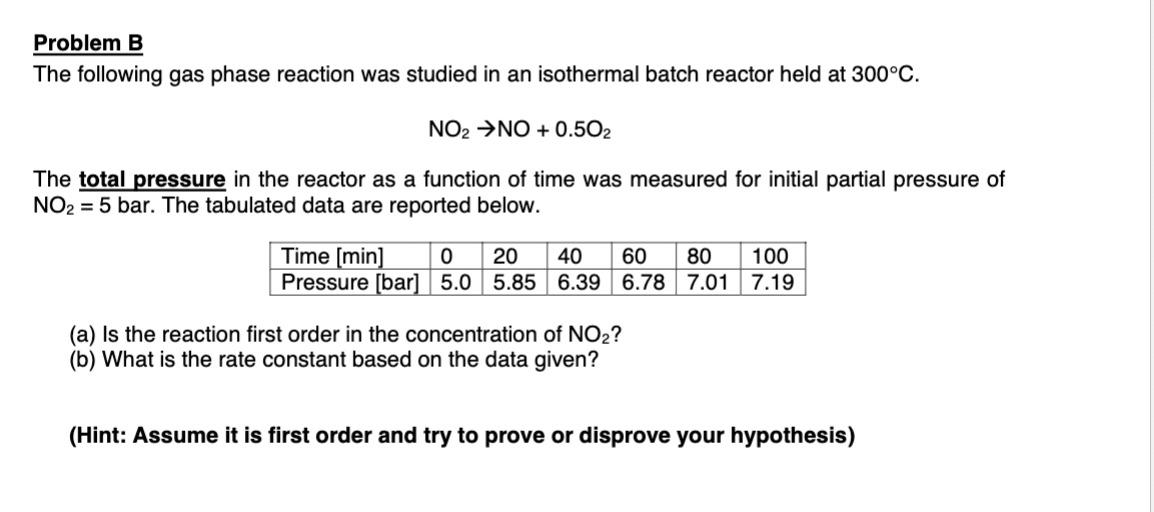
Question: Please answer all sub-questions. Thank you. Problem B The following gas phase reaction was studied in an isothermal batch reactor held at 300C. NO2NO+0.5O2 The
 Please answer all sub-questions. Thank you.
Please answer all sub-questions. Thank you.
Problem B The following gas phase reaction was studied in an isothermal batch reactor held at 300C. NO2NO+0.5O2 The total pressure in the reactor as a function of time was measured for initial partial pressure NO2=5 bar. The tabulated data are reported below. (a) Is the reaction first order in the concentration of NO2 ? (b) What is the rate constant based on the data given? (Hint: Assume it is first order and try to prove or disprove your hypothesis)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


