Question: Please answer all the missing yellow boxes and the 4 questions, showing the work to understand. thank you!! Empirical Formula Data/Results Table 1. Determination of
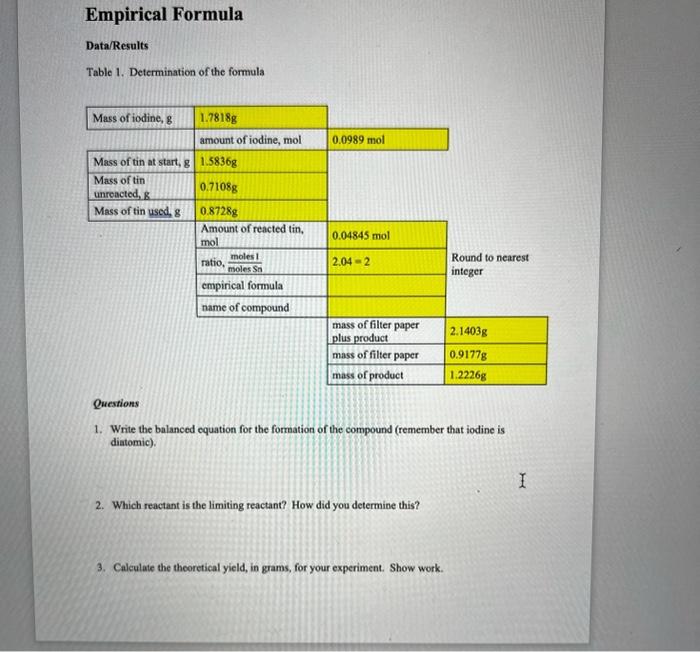
Empirical Formula Data/Results Table 1. Determination of the formula Questions 1. Write the balanced equation for the formation of the compound (remember that iodine is diatomic). 2. Which reactant is the limiting reactant? How did you determine this? 3. Calculate the theoretical yield, in grams, for your experiment. Show work. 4. Calculate the percentage yield for your reaction. Show work. Abstract: Please write an abstract for this experiment. Empirical Formula Data/Results Table 1. Determination of the formula Questions 1. Write the balanced equation for the formation of the compound (remember that iodine is diatomic). 2. Which reactant is the limiting reactant? How did you determine this? 3. Calculate the theoretical yield, in grams, for your experiment. Show work. 4. Calculate the percentage yield for your reaction. Show work. Abstract: Please write an abstract for this experiment
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


