Question: please answer all three :) 1. What is indicated by a large K eq? A solution is saturated b. Products are favored c. Reactants are


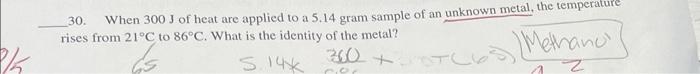
1. What is indicated by a large K eq? A solution is saturated b. Products are favored c. Reactants are favored d. The reaction is endothermic: 25. If Keq=1.75105 for a reaction, which of the following would be true? 2. Reactants are favored at equilibrium b. Products are favored at equilibrium c. Reactants and products are favored equally d. The reaction procecds very rapidly 30. When 300J of heat are applied to a 5.14 gram sample of an unknown metal, the temperature rises from 21C to 86C. What is the identity of the metal
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


