Question: please answer all three, thank you! Nitrogen. N3, is soluble in blood and can cause intoxication at sufficient concentration. For this reason, the U.S. Navy

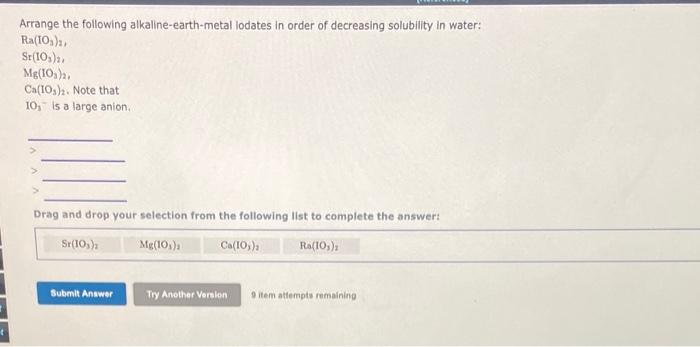
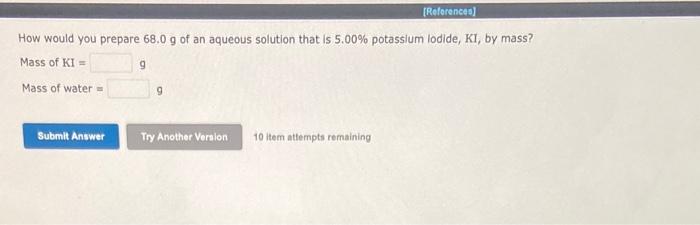
Nitrogen. N3, is soluble in blood and can cause intoxication at sufficient concentration. For this reason, the U.S. Navy advises divers using compressed air not to go below 125 . feet. The total pressure at this depth is 4.79 atm. If the solubility of nitrogen at 1.00atm is 1.761019/100mL of water, and the mole percent of nitrogen in air is 78 . 1 , what is the solubility of nitrogen in water from air at 4.79 atm? Solubility=of/100mL. 10 itaris atienpte ismaliniting Arrange the following alkaline-earth-metal lodates in order of decreasing solubility in water: Ra(IO3)2,Sr(IO3)2,Mg(IO3)2,Ca(IO3)2.Notethat IO3is a large anion. Drag and drop your selection from the following list to complete the answer: How would you prepare 68.0g of an aqueous solution that is 5.00% potassium lodide, KI, by mass? Mass of KI= 9 Mass of water = 9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


