Question: please answer both parts, if not going to answer both please dont answer question!! The concentration of X in an uniknown solution is to be

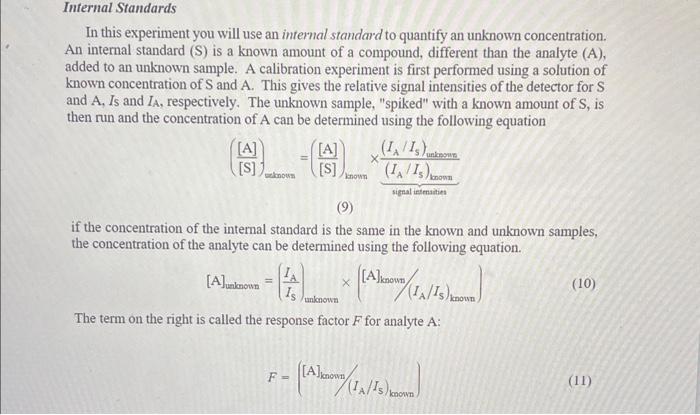
The concentration of X in an uniknown solution is to be determined using the internal standard method. The internal standard Y is present in all salutions at a concentration of 1,8010 M. If a preliminary experiment, a solution containing 9.54102MX and the internal standard resulted in peak areas of 1374 and 1334 for X and Y, respectively. Whut is the response factor F far andivte X? Noge: Use the definition for response factor Fin the experiment handout on page 7. This is different than that in the Harris textbeok which is actually as relative response factor. Tres 2/20 Prevous. Tries An unknawn samble is run and the areas of the X and Y peaks are 5520 and 1304 , respectively. What is the concentration of X in this aample? In this experiment you will use an internal standard to quantify an unknown concentration. An internal standard (S) is a known amount of a compound, different than the analyte (A), added to an unknown sample. A calibration experiment is first performed using a solution of known concentration of S and A. This gives the relative signal intensities of the detector for S and A,Is and IA, respectively. The unknown sample, "spiked" with a known amount of S, is then run and the concentration of A can be determined using the following equation ([S][A])ahnoun=([S][A])inownsignalintmation(IA/IS)brown(IA/IS)uninouno (9) if the concentration of the internal standard is the same in the known and unknown samples, the concentration of the analyte can be determined using the following equation. [A]uaknown=(ISIA)unknown([A]known/(IA/IS)known) The term on the right is called the response factor F for analyte A : F=([A]known/(IA/I5)known) The concentration of X in an uniknown solution is to be determined using the internal standard method. The internal standard Y is present in all salutions at a concentration of 1,8010 M. If a preliminary experiment, a solution containing 9.54102MX and the internal standard resulted in peak areas of 1374 and 1334 for X and Y, respectively. Whut is the response factor F far andivte X? Noge: Use the definition for response factor Fin the experiment handout on page 7. This is different than that in the Harris textbeok which is actually as relative response factor. Tres 2/20 Prevous. Tries An unknawn samble is run and the areas of the X and Y peaks are 5520 and 1304 , respectively. What is the concentration of X in this aample? In this experiment you will use an internal standard to quantify an unknown concentration. An internal standard (S) is a known amount of a compound, different than the analyte (A), added to an unknown sample. A calibration experiment is first performed using a solution of known concentration of S and A. This gives the relative signal intensities of the detector for S and A,Is and IA, respectively. The unknown sample, "spiked" with a known amount of S, is then run and the concentration of A can be determined using the following equation ([S][A])ahnoun=([S][A])inownsignalintmation(IA/IS)brown(IA/IS)uninouno (9) if the concentration of the internal standard is the same in the known and unknown samples, the concentration of the analyte can be determined using the following equation. [A]uaknown=(ISIA)unknown([A]known/(IA/IS)known) The term on the right is called the response factor F for analyte A : F=([A]known/(IA/I5)known)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


